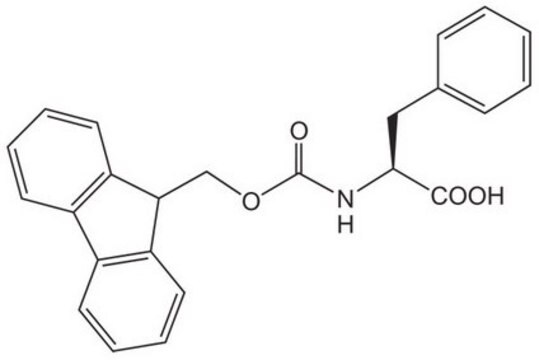
8.55017
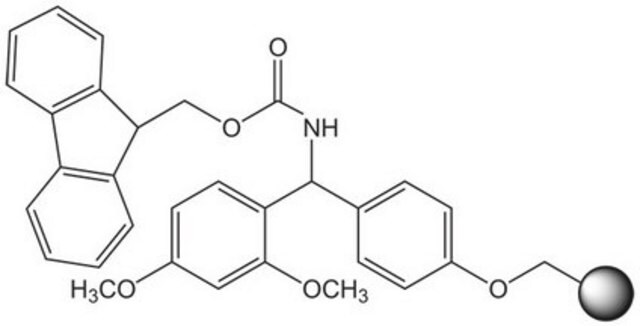
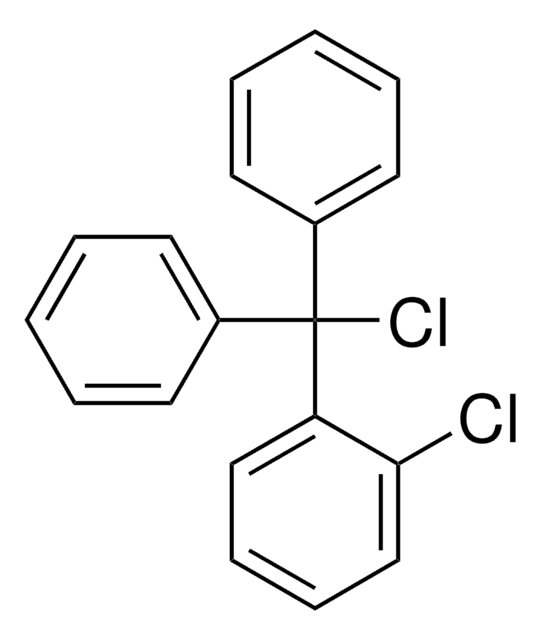
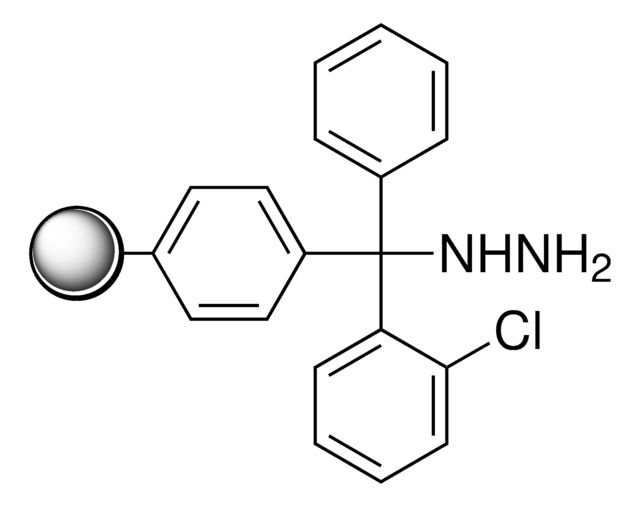
2-Chlorotrityl chloride resin
100-200 mesh, 1% DVB, Novabiochem®
Synonym(s):
2-Chlorotrityl chloride resin
About This Item
Recommended Products
product name
2-Chlorotrityl chloride resin (100-200 mesh), 1% DVB, Novabiochem®
Quality Level
product line
Novabiochem®
form
beads
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
storage temp.
2-30°C
Related Categories
General description
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis ResinsLiterature references
[1] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3947.
[2] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3943.
[3] K. Barlos, et al. (1991) Angew. Chem. Int. Ed. Engl., 30, 590.
[4] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 37, 513.
[5] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 38, 562.
[6] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 38, 555.
[7] H. Wenschuh, et al. in ′Peptides 1994, Proc. 23rd European Peptide Symposium′, H. Maia (Eds), ESCOM, Leiden, 1995, pp. 287.
[8] H. Wenschuh, et al. (1995) J. Org. Chem., 60, 405.
[9] B. B. Shankar, et al. (1998) Tetrahedron Lett., 39, 2447.
[10] Z. Zhu & B. Mckittrick (1998) Tetrahedron Lett., 39, 7479.
[11] U. Heinelt, et al. (2001) Bioorg. Med. Chem. Lett., 11, 227.
[12] K. J. Elgie, et al. (2000) Tetrahedron Lett., 41, 2753.
[13] S. Batra, et al. (2000) Tetrahedron Lett., 41, 5971.
[14] M. Cardno, et al. (1995) J. Chem. Soc., Chem. Commun., 2163.
[15] I. A. Nash, et al. (1996) Tetrahedron Lett., 37, 2625.
[16] W. J. Hoekstra, et al. (1997) Tetrahedron Lett., 38, 2629.
[17] J. Perumattan, et al. (1998) Mol. Div., 3, 121.
[18] J. J. McNally, et al. (1998) Tetrahedron Lett., 39,967.
[19] M. A. Youngman & S. L. Dax (1997) Tetrahedron Lett., 38, 6347.
[20] A. Bernhardt, et al. (1997) J. Peptide Res., 50, 143.
[21] S. L. Mellor, et al. (1997) Tetrahedron Lett., 38, 3311.
[22] M. M. Meloni & M. Taddei (2001) Org. Lett., 3, 337.
[23] R. Bollhagen, et al. (1994) J. Chem. Soc ., Chem. Commun., 2559.
Application
- Efficient Synthesis of Protein Mimics by Sequential Native Chemical Ligation: This study utilized 2-Chlorotrityl chloride resin (100-200 mesh, 1% DVB) for solid-phase peptide synthesis, highlighting its effective use in complex peptide assembly processes. (Kruijtzer & Liskamp).
Linkage
Analysis Note
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Ala-Leu loaded resin): 1.00 - 1.80 mmol/g
Swelling Volume (in CH₂Cl₂): 2.0 - 5.0 ml/g
Total swelling volume acc. Houben Weyl (in CH2Cl2): ≥ 4.2 ml/g
The polymer matrix is copoly (styrene-1 % DVB) 100 - 200 mesh.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Novabiochem® offers a wide range of linkers and derivatized resins for Fmoc solid-phase peptide synthesis with specialized protocols.
Protocols
Review various resins like Merrifield, trityl-based, and hydroxymethyl-functionalized for peptide immobilization for diverse applications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service