63176
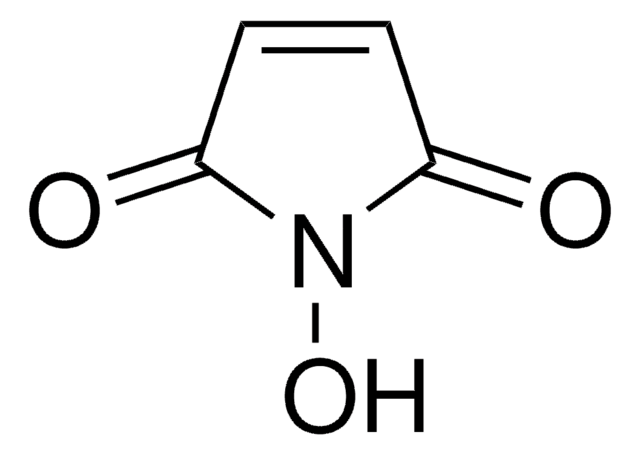
6-Maleimidohexanoic acid
≥98.0% (HPLC)
Synonym(s):
6-Maleimidocaproic acid, N-Maleoyl-6-aminocaproic acid, N-(5-Carboxypentyl)maleimide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H13NO4
CAS Number:
Molecular Weight:
211.21
Beilstein:
1532405
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥98.0% (HPLC)
mp
86-91 °C
storage temp.
room temp
SMILES string
OC(=O)CCCCCN1C(=O)C=CC1=O
InChI
1S/C10H13NO4/c12-8-5-6-9(13)11(8)7-3-1-2-4-10(14)15/h5-6H,1-4,7H2,(H,14,15)
InChI key
WOJKKJKETHYEAC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
6-Maleimidohexanoic acid may be used as a spacer in the construction of drug and other types of bioconjugates. 6-Maleimidohexanoic acid is used with N-hydroxysuccinimide ester as a bifunctional cross-linking reagent.
Probe for thiol groups (SH-groups) in membrane proteins.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shinya Kida et al.
Chemical & pharmaceutical bulletin, 55(4), 685-687 (2007-04-06)
6-maleimidohexanoic acid N-hydroxysuccinimide ester has been used widely for preparation of enzyme immunoconjugates as a unique heterobifunctional cross-linking reagent. Its heterobifunctional reactivity is good, but its ester portion hydrolyzes easily in the presence of water. Several 6-maleimidohexanoic acid active esters
Ralph Rahme et al.
Journal of neurosurgery, 121(6), 1354-1358 (2014-09-27)
The role of endovascular therapy in patients with acute ischemic stroke and a solitary M2 occlusion remains unclear. Through a pooled analysis of 3 interventional stroke trials, the authors sought to analyze the impact of successful early reperfusion of M2
Lauren L Jantzie et al.
Cerebral cortex (New York, N.Y. : 1991), 25(2), 482-495 (2013-09-21)
The pathophysiology of perinatal brain injury is multifactorial and involves hypoxia-ischemia (HI) and inflammation. N-methyl-d-aspartate receptors (NMDAR) are present on neurons and glia in immature rodents, and NMDAR antagonists are protective in HI models. To enhance clinical translation of rodent
Mitsuko Maeda et al.
Bioorganic & medicinal chemistry letters, 15(3), 621-624 (2005-01-25)
The adenovirus vector is a promising carrier for the efficient transfer of genes into cells via the coxackie-adenovirus receptor (CAR) and integrins (alphavbeta3 and alphavbeta5). The clinical use of the adenovirus vector remains problematic however. Successful administration of this vector
S S Ghosh et al.
Bioconjugate chemistry, 1(1), 71-76 (1990-01-01)
Two general methods which exploit the reactivity of sulfhydryl groups toward maleimides are described for the synthesis of oligonucleotide-enzyme conjugates for use as nonradioisotopic hybridization probes. In the first approach, 6-maleimidohexanoic acid succinimido ester was used to couple 5'-thiolated oligonucleotide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service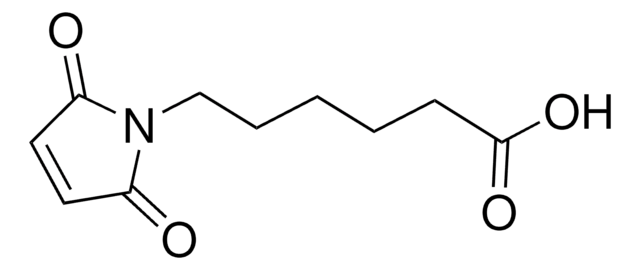



![O-[2-(Boc-amino)ethyl]-O′-(2-maleimidoethyl)ethylene glycol ≥96.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/270/188/cd3145b1-0ad3-409b-85c2-73937e007f04/640/cd3145b1-0ad3-409b-85c2-73937e007f04.png)