95137
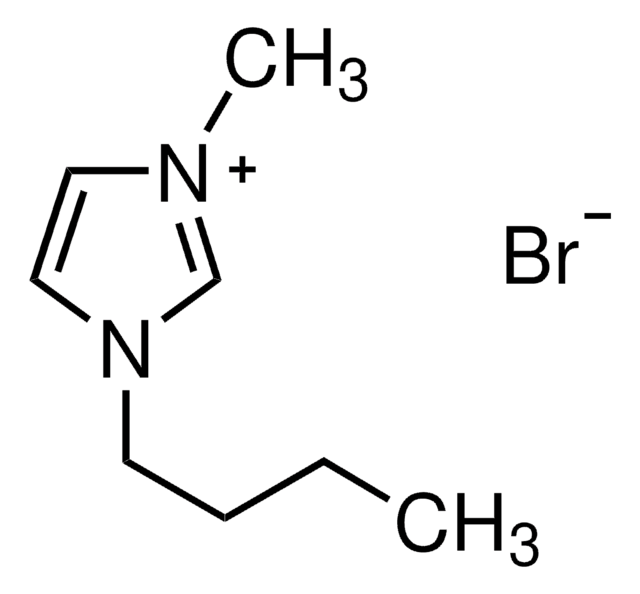
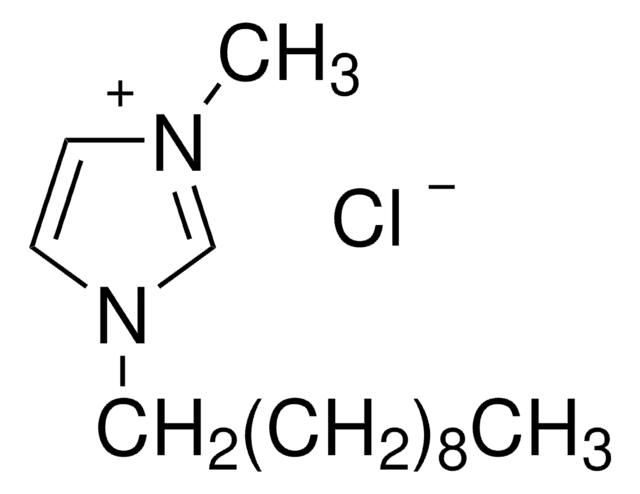
1-Butyl-3-methylimidazolium bromide
>97.0% (HPLC)
Synonym(s):
BMIMBr
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H15BrN2
CAS Number:
Molecular Weight:
219.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
>97.0% (HPLC)
form
crystals
impurities
≤1% water
SMILES string
[Br-].CCCCn1cc[n+](C)c1
InChI
1S/C8H15N2.BrH/c1-3-4-5-10-7-6-9(2)8-10;/h6-8H,3-5H2,1-2H3;1H/q+1;/p-1
InChI key
KYCQOKLOSUBEJK-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
1-Butyl-3-methylimidazolium bromide is a neutral ionic liquid.
Application
BMIMBr undergoes gelation with gelatin to form ion jelly, a quasi-solid material for use in chemoresistive gas sensors. It can be used as a solvent for the preparation of 1,2,4,5-substituted imidazoles.
Other Notes
Ionic liquid used in a Heck reaction
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L. Xu et al. et al.
Organometallics, 19, 1123-1123 (2000)
Teng-Hui Wang et al.
International journal of molecular sciences, 22(2) (2021-01-23)
Mixtures of polyethylene oxide (PEO, M.W.~900,000) and imidazolium ionic liquids (ILs) are studied using high-pressure Fourier-transform infrared spectroscopy. At ambient pressure, the spectral features in the C-H stretching region reveal that PEO can disturb the local structures of the imidazolium
Phase diagrams for the aqueous two-phase ternary system containing the ionic liquid 1-butyl-3-methylimidazolium bromide and tri-potassium citrate at T=(278.15, 298.15, and 318.15) K
Zafarani-Moattar, Mohammed Taghi, and Sholeh Hamzehzadeh
Journal of Chemical and Engineering Data, 54.3, 833-841 (2008)
Apparent molar volume and isentropic compressibility of ionic liquid 1-butyl-3-methylimidazolium bromide in water, methanol, and ethanol at T=(298.15 to 318.15) K
Zafarani-Moattar, Mohammed Taghi, and Hemayat Shekaari
The Journal of Chemical Thermodynamics, 37.10, 1029-1035 (2005)
Melting and freezing behaviors of prototype Ionic Liquids, 1-Butyl-3-methylimidazolium bromide and its chloride, studied by using a nano-Watt differential scanning calorimeter
Nishikawa, Keiko, et al.
The Journal of Physical Chemistry B, 111.18, 4894-4900 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service