D0632
DL-Dithiothreitol
≥98% (HPLC), ≥99.0% (titration)
Synonym(s):
(±)-Dithiothreitol, rac-Dithiothreitol, Dithiothreitol, threo-1,4-Dimercapto-2,3-butanediol, Cleland’s reagent, DTT
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
≥99.0% (titration)
form
powder
reaction suitability
reagent type: reductant
color
white
mp
41-44 °C (lit.)
solubility
H2O: soluble 50 mg/mL, clear, colorless to very faintly yellow
application(s)
general analytical
storage temp.
2-8°C
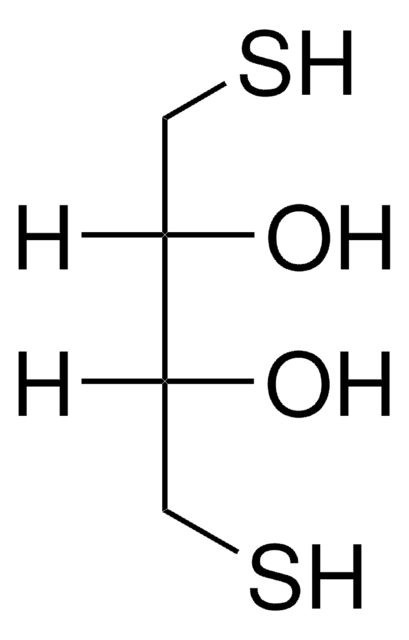
SMILES string
O[C@H](CS)[C@H](O)CS
InChI
1S/C4H10O2S2/c5-3(1-7)4(6)2-8/h3-8H,1-2H2/t3-,4-/m1/s1
InChI key
VHJLVAABSRFDPM-QWWZWVQMSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as one of the reactants in the reduction and alkylation of αs1-Casein, the major allergen of cow′s milk.
- as a component of medium for the demembranation and reactivation of spermatozoa.
- to maintain stability of the enzyme as thiol effectively protects the active sites of the biocatalyst.
- as a reducing agent to test the specificity of the reaction of N-Ethylmaleimide with sulfhydryl groups.
- in proteomics analysis as in-solution protein digestion for mass spectrometry
- as a buffer component for protein quantification, to prepare wash buffer, lysis buffer, sample buffer, and protein elution buffer
Biochem/physiol Actions
Features and Benefits
- High-quality DTT (HPLC≥98%), (titration≥99.0%)
- Suitable for electrophoresis, proteomics analysis
Other Notes
also commonly purchased with this product
comparable product
suggested gloves for splash protection
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Compare columns in resolving medium-sized antibody fragments after digestion with DTT or IdeS using Reversed-Phase Chromatography for analysis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service