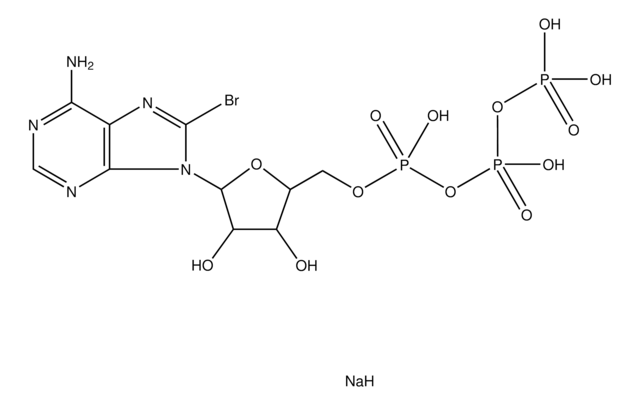
B3131
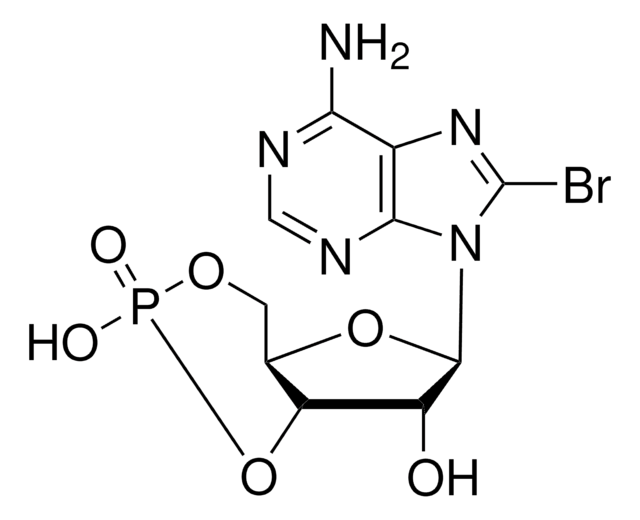
8-Bromoadenosine 5′-monophosphate
≥98%
Synonym(s):
8-Br-AMP
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H13BrN5O7P
CAS Number:
Molecular Weight:
426.12
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥98%
form
powder
solubility
water: 100 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
Nc1ncnc2n(C3OC(COP(O)(O)=O)C(O)C3O)c(Br)nc12
InChI
1S/C10H13BrN5O7P/c11-10-15-4-7(12)13-2-14-8(4)16(10)9-6(18)5(17)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,17-18H,1H2,(H2,12,13,14)(H2,19,20,21)
InChI key
DNPIJKNXFSPNNY-UHFFFAOYSA-N
Related Categories
Application
8-Bromoadenosine 5′′-monophosphate (8-Br-AMP) is an analogue of 5′-AMP useful for receptor mapping studies; as a starting structure for 8-modified 5′-AMP derivatives and for synthesis of poly-8-bromoriboadenylic acid.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K Lesiak et al.
Biochemical and biophysical research communications, 126(2), 917-921 (1985-01-31)
When added to extracts of mouse L cells containing ATP and an energy regenerating system, the 5'-diphosphate of 2-5A core, pp5'A2'p5'A2'p5'A, as well as a bromoadenylate analog, pp5' (br8A)2'p5'(br8A)2'p5'(br8A), can be phosphorylated to the corresponding 5'-triphosphate, ppp5'A2'p5'A2'p5'A and ppp5'(br8A)2'p5'(br8A)2'p5(br8A), respectively.
J O Folayan et al.
Journal of biochemistry, 96(4), 1297-1301 (1984-10-01)
Poly-8-bromoriboadenylic acid was synthesized by the bromination of adenosine-5'-monophosphate to yield 8-bromoadenosine-5'-monophosphate which on conversion to the 5'-diphosphate form was polymerized by polynucleotide phosphorylase (PNPase). The polymer formed a 1:1 hybrid with polyribouridylic acid and the hybrid was found to
Rongkuan Hu et al.
PloS one, 8(8), e73527-e73527 (2013-08-24)
This study is the first to demonstrate that shizukaol D, a natural compound isolated from Chloranthusjaponicus, can activate AMP- activated protein kinase (AMPK), a key sensor and regulator of intracellular energy metabolism, leading to a decrease in triglyceride and cholesterol
Guido Michels et al.
PloS one, 3(1), e1511-e1511 (2008-01-31)
Hyperpolarization-activated, cyclic nucleotide sensitive (HCN) channels underlie the pacemaker current I(f), which plays an essential role in spontaneous cardiac activity. HCN channel subunits (HCN1-4) are believed to be modulated by additional regulatory proteins, which still have to be identified. Using
Gijs Teklenburg et al.
PloS one, 7(3), e32701-e32701 (2012-03-14)
Female mammals inactivate one of their two X-chromosomes to compensate for the difference in gene-dosage with males that have just one X-chromosome. X-chromosome inactivation is initiated by the expression of the non-coding RNA Xist, which coats the X-chromosome in cis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service