All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C26H41N3O5
CAS Number:
Molecular Weight:
475.62
UNSPSC Code:
12352200
NACRES:
NA.32
Recommended Products
Assay
≥98%
Quality Level
solubility
DMSO or DMF: 25 mg/mL
storage temp.
−20°C
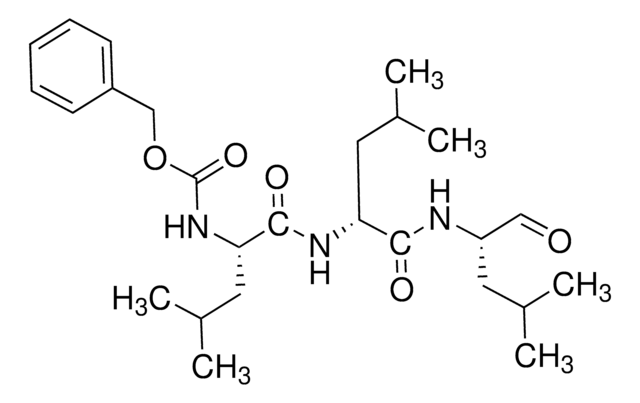
SMILES string
CC(C)C[C@@H](C(N[C@H](C(N[C@H](C=O)CC(C)C)=O)CC(C)C)=O)NC(OCC1=CC=CC=C1)=O
InChI
1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1
InChI key
TZYWCYJVHRLUCT-VABKMULXSA-N
Application
(R)-MG132 has been used in ubiquitination assay and is used as a proteasome inhibitor.
Biochem/physiol Actions
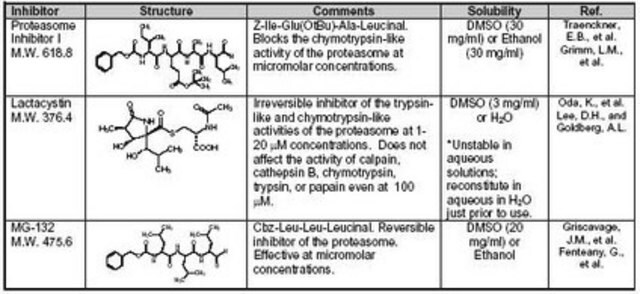
MG132 (carbobenzoxy-Leu-Leu-leucinal) is a tri-peptide aldehyde. It possesses antitumor activity and boosts cytostatic/cytotoxic effects of chemo- and radiotherapy. (R)-MG132 is a potent, membrane-permeable proteasome inhibitor. It can inhibit proteasome activity in lysates of J558L multiple myeloma cells and EMT6 breast cancer cells. The (R)-MG132 stereoisomer is a more effective inhibitor of chymotrypsin-like (ChTL), trypsin-like (TL), and peptidylglutamyl peptide hydrolyzing proteasome (PGPH) activities than the (S)-MG132.
Physical form
crystalline solid or supercooled liquid
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Mad2-Mediated Translational Regulatory Mechanism Promoting S-Phase Cyclin Synthesis Controls Origin Firing and Survival to Replication Stress.
Gay S, et al.
Molecular Cell, 70(4), 628-638 (2018)
Alternative promotion and suppression of metastasis by JNK2 governed by its phosphorylation.
Hu S, et al.
Oncotarget, 8(34), 56569-56569 (2017)
Studies of the synthesis of all stereoisomers of MG-132 proteasome inhibitors in the tumor targeting approach.
Mroczkiewicz M, et al.
Journal of Medicinal Chemistry, 53(4), 1509-1518 (2010)
Activation of anaphase-promoting complex by p53 induces a state of dormancy in cancer cells against chemotherapeutic stress.
Dai Y, et al.
Oncotarget, 7(18), 25478-25478 (2016)
Determination of H-ATPase Activity in Arabidopsis Guard Cell Protoplasts through H-pumping Measurement and H-ATPase Quantification.
Yamauchi S and Ken-ichiro S
Plant Physiology (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service