109606
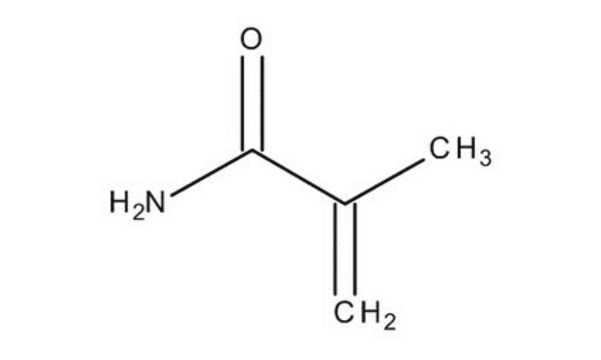
Methacrylamide
98%
Synonym(s):
2-Methylacrylamide, 2-Methylpropenamide, Methacrylic acid amide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2C=C(CH3)CONH2
CAS Number:
Molecular Weight:
85.10
Beilstein:
605397
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
solid
mp
106-110 °C (lit.)
SMILES string
CC(=C)C(N)=O
InChI
1S/C4H7NO/c1-3(2)4(5)6/h1H2,2H3,(H2,5,6)
InChI key
FQPSGWSUVKBHSU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Methacrylamide is a functional monomer used inthe preparation of thermo-sensitive hydrogels, polymer latexes, and self-etchingprimers with a longer shelf life. It can also be used to prepare dispersionsuspensions to synthesize biocompatible HA/sol-gel glass mixtures.
Application
- Synthesis of non-fouling poly brushes by photoinduced SET-LRP: This study highlights the use of photoinduced SET-LRP for the polymerization of methacrylamide, emphasizing its efficiency and the quality of the resulting polymers for non-fouling applications (M Vorobii, A de los Santos Pereira, 2015).
- Hydrolytic stability of methacrylamide and methacrylate in gelatin methacryloyl: The study investigates the hydrolytic stability of methacrylamide within gelatin methacryloyl, highlighting its stability and potential in biomedical applications (J Zheng, M Zhu, G Ferracci, NJ Cho, 2018).
- Two-step mechanisms of tumor selective delivery of N-(2-hydroxypropyl) methacrylamide copolymer conjugated with pirarubicin via an acid-cleavable linkage: This paper discusses the development of a copolymer conjugate for targeted cancer therapy, showcasing a two-step mechanism for enhanced drug delivery (H Nakamura, T Etrych, P Chytil, M Ohkubo, 2014).
- Backbone Degradable N-(2-Hydroxypropyl)methacrylamide Copolymer Conjugates with Gemcitabine and Paclitaxel: The research focuses on degradable copolymer conjugates for delivering cancer therapeutics, noting significant effects on tumor reduction and highlighting the impact of molecular weight (J Yang, R Zhang, H Pan, Y Li, Y Fang, 2017).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT RE 2 - STOT SE 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sinoj Abraham et al.
Biomacromolecules, 12(10), 3567-3580 (2011-09-07)
Nonfouling polymer architectures are considered important to the successful implementation of many biomaterials. It is thought that how these polymers induce conformational changes in proteins upon adsorption may dictate the fate of the device being utilized. Herein, oxidized silicon nanoparticles
Hoyong Chung et al.
Biomacromolecules, 12(2), 342-347 (2010-12-25)
We present a study on the effects of cross-linking on the adhesive properties of bio-inspired 3,4-dihydroxyphenylalanine (DOPA). DOPA has a unique catechol moiety found in adhesive proteins in marine organisms, such as mussels and polychaete, which results in strong adhesion
Ali Ghadban et al.
Biomacromolecules, 13(10), 3108-3119 (2012-08-30)
Ionotropic gelation is particularly appealing for the formation of hydrogels because it takes place under mild conditions, is not thermoreversible, and does not involve toxic chemicals. A well-known example is the gelation of alginate in the presence of calcium ions
Elizabeth F Irwin et al.
Biomaterials, 32(29), 6912-6919 (2011-07-22)
We have developed a synthetic polymer interface for the long-term self-renewal of human embryonic stem cells (hESCs) in defined media. We successfully cultured hESCs on hydrogel interfaces of aminopropylmethacrylamide (APMAAm) for over 20 passages in chemically-defined mTeSR™1 media and demonstrated
Sascha Engelhardt et al.
Biofabrication, 3(2), 025003-025003 (2011-05-13)
Two-photon polymerization (TPP) offers the possibility of creating artificial cell scaffolds composed of micro- and nanostructures with spatial resolutions of less than 1 µm. For use in tissue engineering, the identification of a TPP-processable polymer that provides biocompatibility, biofunctionality and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service