123056
4-(2-Aminoethyl)aniline
97%
Synonym(s):
4-Aminophenethylamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
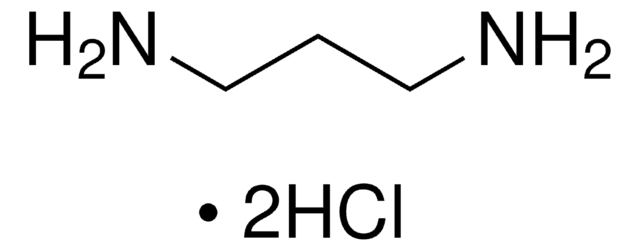
H2NC6H4CH2CH2NH2
CAS Number:
Molecular Weight:
136.19
Beilstein:
1099913
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.591 (lit.)
bp
103 °C/0.3 mmHg (lit.)
mp
28-31 °C (lit.)
density
1.034 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
NCCc1ccc(N)cc1
InChI
1S/C8H12N2/c9-6-5-7-1-3-8(10)4-2-7/h1-4H,5-6,9-10H2
InChI key
LNPMZQXEPNWCMG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-(2-Aminoethyl)aniline undergoes coupling with carbohydrates by reductive amination to yield modified carbohydrates.
Application
4-(2-Aminoethyl)aniline has been used in chemical modification of silk fibroin to tailor the overall hydrophilicity and structure of silk. It has been used as reagent in polycondensation reactions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Amanda R Murphy et al.
Biomaterials, 29(19), 2829-2838 (2008-04-18)
A simple chemical modification method using diazonium coupling chemistry was developed to tailor the structure and hydrophilicity of silk fibroin protein. The extent of modification using several aniline derivatives was characterized using UV-vis and 1H NMR spectroscopies, and the resulting
Polymer Journal, 23, 1511-1511 (1991)
Isabella A Lobo et al.
Physical chemistry chemical physics : PCCP, 14(25), 9219-9229 (2012-05-31)
The neurotransmitter analogue p-aminophenethylamine (APEA) illustrates many of the pitfalls and challenges associated with spectroscopic and conformational analysis of flexible molecules. The combined experimental-theoretical study presented here resolves a long-standing controversy over its conformational energetic preferences. Jet-cooled resonance enhanced two-photon
Macromolecules, 27, 1289-1289 (1994)
M Reyes-Parada et al.
Biochemical pharmacology, 47(8), 1365-1371 (1994-04-20)
The in vitro and ex vivo monoamine oxidase (MAO) inhibitory effects of (+/-)4-dimethylamino-alpha-methyl-phenethylamine (4-DMAA) and (+/-)4-methylamino-alpha-methyl-phenethylamine (4-MAA) were reassessed, in comparison with the previously unstudied achiral parent compound, 4-dimethyl-aminophenethylamine (4-DMAPEA) and with a salt of 4-DMAA enriched in the levo
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service