125415
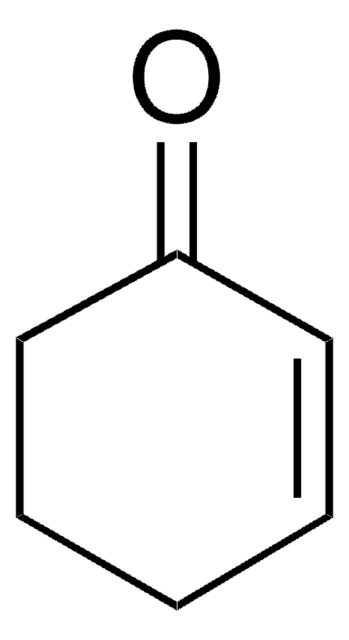
1,4-Cyclohexadiene
97%
Synonym(s):
1,4-Dihydrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H8
CAS Number:
Molecular Weight:
80.13
Beilstein:
1900733
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
contains
~0.1% hydroquinone as stabilizer
impurities
3% benzene
refractive index
n20/D 1.472 (lit.)
bp
88-89 °C (lit.)
density
0.847 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C1C=CCC=C1
InChI
1S/C6H8/c1-2-4-6-5-3-1/h1-2,5-6H,3-4H2
InChI key
UVJHQYIOXKWHFD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,4-Cyclohexadiene is an effective hydrogen donor for catalytic hydrogenation reactions. It can rapidly replace benzyl groups of N-benzyloxycarbamates, benzyl esters, benzyl ethers and benzyl amines with hydrogen. It forms benzene at elevated temperatures in the presence of a ruthenium(II)-triphenylphosphine catalyst.
Application
1,4-Cyclohexadiene (1,4-CHD) was used to study the formation of parent ion from heavy fragmentation of 1,4-CHD on irradiation with a high-intensity laser pulse.
Useful for the reduction of radical intermediates formed in electron-transfer mediated ring-opening reactions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1A - Flam. Liq. 2 - Muta. 1B - STOT RE 2
Target Organs
Blood
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
19.4 °F - closed cup
Flash Point(C)
-7 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Organometallics, 25, 5456-5456 (2006)
A key factor in parent and fragment ion formation on irradiation with an intense femtosecond laser pulse.
Harada H, et al.
Chemical Physics Letters, 342(5), 563-570 (2001)
Vladimir S Petrović et al.
Physical review letters, 108(25), 253006-253006 (2012-09-26)
We report the first study of UV-induced photoisomerization probed via core ionization by an x-ray laser. We investigated x-ray ionization and fragmentation of the cyclohexadiene-hexatriene system at 850 eV during the ring opening. We find that the ion-fragmentation patterns evolve
Monika Ali Khan et al.
Chemical communications (Cambridge, England), 47(1), 215-217 (2010-08-24)
A cyclohexadiene ligand prepared by microbial arene 1,2-dihydroxylation undergoes spontaneous rearrangement upon complexation to tricarbonyliron(0). Subsequent iron removal affords a novel route to formal arene 2,3-dihydroxylation products enantiomeric to those obtainable by direct microbial arene oxidation.
Beni Camacho-Pérez et al.
Journal of environmental management, 95 Suppl, S306-S318 (2011-10-14)
The scope of this paper encompasses the following subjects: (i) aerobic and anaerobic degradation pathways of γ-hexachlorocyclohexane (HCH); (ii) important genes and enzymes involved in the metabolic pathways of γ-HCH degradation; (iii) the instrumental methods for identifying and quantifying intermediate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)