132551
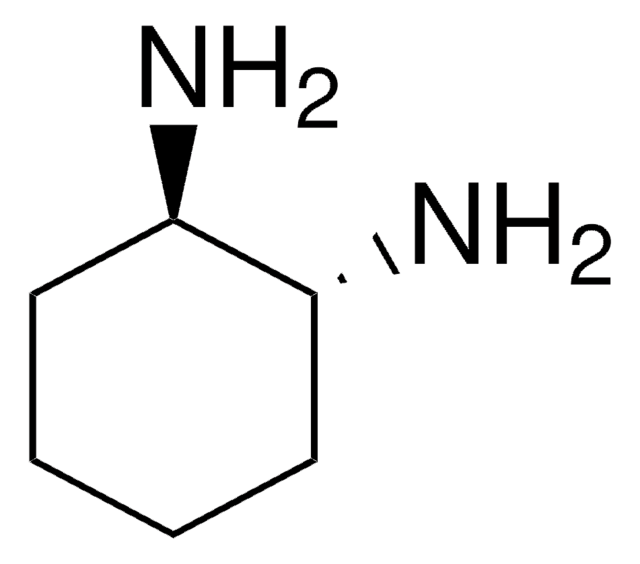
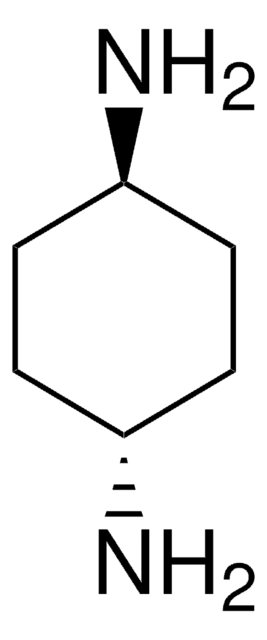
1,2-Diaminocyclohexane, mixture of cis and trans
99%
Synonym(s):
1,2-Cyclohexanediamine, DHC 99
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H10(NH2)2
CAS Number:
Molecular Weight:
114.19
Beilstein:
506142
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
0.4 mmHg ( 20 °C)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.49 (lit.)
bp
92-93 °C/18 mmHg (lit.)
density
0.931 g/mL at 25 °C (lit.)
SMILES string
NC1CCCCC1N
InChI
1S/C6H14N2/c7-5-3-1-2-4-6(5)8/h5-6H,1-4,7-8H2
InChI key
SSJXIUAHEKJCMH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1,2-Diaminocyclohexane undergoes non-templated reaction with homochiral as well as the racemic form of trans-1,2-diaminocyclohexane with terephthaldehyde to yield (3+3)-cyclocondensed molecular triangles. It acts as ligand and forms organotin complexes, having potential applications as metal-based antitumour drugs.
Application
1,2-Diaminocyclohexane was used in the synthesis of chiral ruthenium(IV)-oxo complexes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
158.0 °F - closed cup
Flash Point(C)
70 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chiral ruthenium (IV)-oxo complexes. Structure, reactivities of [Ru (terpy)(Nn N) O]2+Nn N= N, N, N', N'-tetramethyl-1, 2-diaminocyclohexane) and [Ru (Me3 tacn)(cbpy) O]2+ (cbpy=(-)-3, 3'-[(4 S-trans)-1, 3-dioxolane-4, 5-dimethyl]-2, 2'-bipyridine).
Cheng WC, et al.
Inorgorganica Chimica Acta, 242(1), 105-113 (1996)
J J Bonire et al.
Journal of inorganic biochemistry, 83(2-3), 217-221 (2001-03-10)
Platinum compounds containing the ligand 1,2-diaminocyclohexane (DACH) such as tetraplatin [PtCl4(DACH)] have been found to be active in cisplatin-resistant tumour models. In an attempt to develop novel metal-based drugs with a different therapeutic profile to cisplatin, we have synthesised a
(3+ 3)-Cyclocondensation of the enantiopure and racemic forms of trans-1, 2-diaminocyclohexane with terephthaldehyde. Formation of diastereomeric molecular triangles and their stereoselective solid-state stacking into microporous chiral columns.
Chadim M, et al.
Tetrahedron Asymmetry, 12(1), 127-133 (2001)
Kenji Yoshikawa et al.
Bioorganic & medicinal chemistry, 17(24), 8206-8220 (2009-11-04)
A series of cis-1,2-diaminocyclohexane derivatives were synthesized with the aim of optimizing previously disclosed factor Xa (fXa) inhibitors. The exploration of 5-6 fused rings as alternative S1 moieties resulted in two compounds which demonstrated improved solubility and reduced food effect
Hidekazu Yamada et al.
Journal of the American Chemical Society, 134(17), 7250-7253 (2012-04-18)
Optically active amidine dimer strands having a variety of chiral and achiral linkers with different stereostructures are synthesized and used as templates for diastereoselective imine-bond formations between two achiral carboxylic acid monomers bearing a terminal aldehyde group and racemic 1,2-cyclohexanediamine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service