All Photos(1)
About This Item
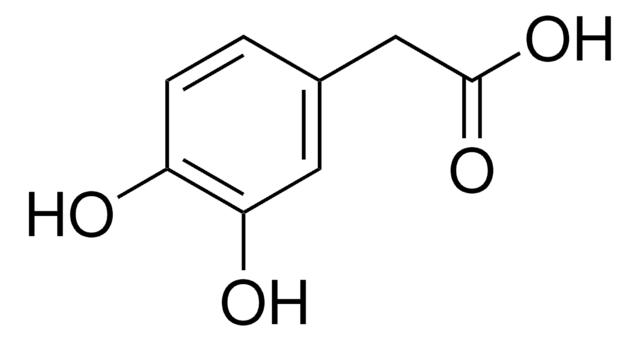
Linear Formula:
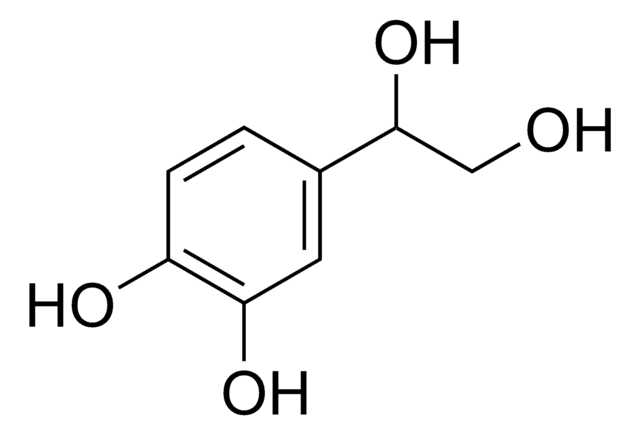
(HO)2C6H3CH(OH)CO2H
CAS Number:
Molecular Weight:
184.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
136-137 °C (dec.) (lit.)
functional group
carboxylic acid
hydroxyl
SMILES string
OC(C(O)=O)c1ccc(O)c(O)c1
InChI
1S/C8H8O5/c9-5-2-1-4(3-6(5)10)7(11)8(12)13/h1-3,7,9-11H,(H,12,13)
InChI key
RGHMISIYKIHAJW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Metabolite of norepinephrine.
Application
DL-3,4-Dihydroxymandelic acid was used in the simultaneous analysis of 4-hydroxy-3-methoxymandelic acid and 4-hydroxy- 3-methoxyphenylacetic acid in urine. It was also used to study the changes in body temperature.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Kawamura et al.
Journal of the autonomic nervous system, 66(3), 145-148 (1997-12-24)
After norepinephrine (NE) is deaminated by monoamine oxidase (MAO), the aldehyde formed is either metabolized to 3,4-dihydroxy-mandelic acid (DHMA) by aldehyde dehydrogenase or is converted to 3,4-dihydroxyphenylglycol (DHPG) by aldehyde or aldose reductase. The present study examined the effects of
J Y Li et al.
Analytical biochemistry, 190(2), 354-359 (1990-11-01)
A quantitative assay for the diphenol oxidase activity of tyrosinase (EC 1.14.18.1) using high-pressure liquid chromatography with electrochemical detection is described. The assay is based on the observation (M. Sugumaran, 1986, Biochemistry 25, 4489-4492) that tyrosinase catalyzes the oxidative decarboxylation
S J Soldin et al.
Clinical chemistry, 26(2), 291-294 (1980-02-01)
We describe a rapid, reliable "high-performance" liquid-chromatographic method of simultaneously analyzing for 4-hydroxy-3-methoxymandelic acid (I) and 4-hydroxy-3-methoxyphenylacetic acid (II) in urine. Paired-ion chromatography and amperometric detection are used in the method. A 5-mL aliquot of urine is adjusted to pH
Mechanistic studies on tyrosinase-catalysed oxidative decarboxylation of 3,4-dihydroxymandelic acid.
M Sugumaran et al.
The Biochemical journal, 281 ( Pt 2), 353-357 (1992-01-15)
Mushroom tyrosinase, which is known to convert a variety of o-diphenols into o-benzoquinones, has been shown to catalyse an unusual oxidative decarboxylation of 3,4-dihydroxymandelic acid to 3,4-dihydroxybenzaldehyde [Sugumaran (1986) Biochemistry 25, 4489-4492]. The mechanism of this reaction was re-investigated. Although
Andrea E Schwaninger et al.
Toxicology letters, 202(2), 120-128 (2011-02-08)
3,4-Methylenedioxymethamphetamine (MDMA, Ecstasy) is excreted in human urine mainly as conjugates of its metabolites 3,4-dihydroxymethamphetamine (DHMA) and 4-hydroxy-3-methoxymethamphetamine (HMMA). The glucuronidation kinetics of HMMA showed high capacities, but also high K(m) values, unlikely to be reached after recreational user's doses.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service