159441
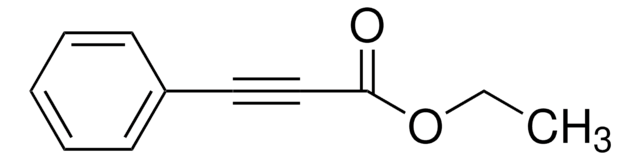
Diethyl acetylenedicarboxylate
95%
Synonym(s):
Diethyl 2-butynedioate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5OCOC≡CCOOC2H5
CAS Number:
Molecular Weight:
170.16
Beilstein:
743166
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.443 (lit.)
bp
107-110 °C/11 mmHg (lit.)
density
1.063 g/mL at 25 °C (lit.)
functional group
ester
storage temp.
2-8°C
SMILES string
CCOC(=O)C#CC(=O)OCC
InChI
1S/C8H10O4/c1-3-11-7(9)5-6-8(10)12-4-2/h3-4H2,1-2H3
InChI key
STRNXFOUBFLVIN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Diethyl acetylenedicarboxylate is a protein cross-linker.
Diethyl acetylenedicarboxylate is used as a Michael acceptor for O-vinyl oximes synthesis, and is used in the nucleophilic addition reaction.
Diethyl acetylenedicarboxylate is used as a Michael acceptor for O-vinyl oximes synthesis, and is used in the nucleophilic addition reaction.
Application
Diethyl acetylenedicarboxylate was used in the synthesis of:
- 3,4,5-trisubstituted 2(5H)-furanone derivatives
- highly functionalized thiazolidinone derivatives
- novel cyclic peroxide glucosides
- 4,11-dimesitylbisanthene, soluble bisanthene derivative, via Diels-Alder reaction
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
201.2 °F - closed cup
Flash Point(C)
94 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
W M Basyouni et al.
Drug research, 65(9), 473-478 (2014-09-11)
A series of 3,4,5-trisubstituted 2(5H)-furanone derivatives was synthesized through one-pot reaction of amines, aldehydes and diethyl acetylenedicarboxylate. Silica sulfuric acid efficiently catalyzes the 3-component reaction to afford the corresponding 2(5H)-furanones in high yields. The synthesized compounds were tested against HEPG2
Di-Zao Li et al.
Journal of Asian natural products research, 11(7), 613-620 (2010-02-26)
Four novel cyclic peroxide glucosides 15a, 15b, 16a, and 16b, optically pure analogs of shuangkangsu (1), which is an anti-virus natural product with an unusual skeleton isolated from the buds of Lonicera japonica Thunb, were first synthesized totally in six
Abdelmadjid Benmohammed et al.
Molecules (Basel, Switzerland), 19(3), 3068-3083 (2014-03-13)
We present herein the synthesis in good yields of two series of highly functionalized thiazolidinone derivatives from the reactions of various 4-phenyl-3-thio-semicarbazones with ethyl 2-bromoacetate and diethyl acetylenedicarboxylate, respectively.
Microwave Induced Stereoselective Synthesis of O--Vinyl Oximes using Acetylenic Esters as Efficient Michael Acceptors
Ankush M et al.
ChemistrySelect, 3, 9464-9468 (2018)
Eric H Fort et al.
Journal of the American Chemical Society, 131(44), 16006-16007 (2009-10-17)
A soluble bisanthene derivative, 4,11-dimesitylbisanthene, has been synthesized in three steps from bianthrone. In hot toluene, this bisanthene undergoes a clean Diels-Alder reaction with diethyl acetylenedicarboxylate to give a rearomatized 1:1 cycloadduct and, more slowly, a rearomatized 2:1 cycloadduct. In
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service