220051
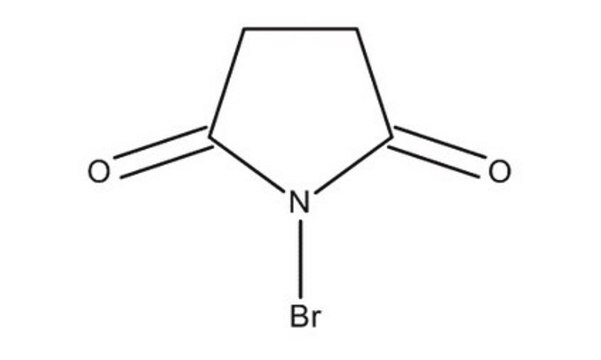
N-Iodosuccinimide
95%
Synonym(s):
1-iodo-pyrrolidine-2,5-dione, 1-iodoazolidine-2,5-dione, NIS, succiniodimide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H4INO2
CAS Number:
Molecular Weight:
224.98
Beilstein:
113917
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
powder
mp
202-206 °C (lit.)
functional group
imide
storage temp.
2-8°C
SMILES string
IN1C(=O)CCC1=O
InChI
1S/C4H4INO2/c5-6-3(7)1-2-4(6)8/h1-2H2
InChI key
LQZMLBORDGWNPD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Highly substituted iodobenzenes prepared via an efficient 2-step process from 1,6-diynes. Used with TFA to chemoselectively hydrolyze thioglycosides to 1-hydroxyglycosides. Synthesis of vinyl sulfones from olefins and benzenesulfinic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Muta. 2 - Skin Irrit. 2 - Skin Sens. 1B
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Canadian Journal of Chemistry, 84, 66-66 (2006)
Yoshihiko Yamamoto et al.
Journal of the American Chemical Society, 128(25), 8336-8340 (2006-06-22)
Highly substituted iodobenzenes were efficiently and regioselectively synthesized from readily available 1,6-diynes via two-step process consisting of silver-catalyzed Csp-H iodination and subsequent ruthenium-catalyzed [2 + 2 + 2] cycloaddition of resultant iododiynes. Some of the obtained iodobenzenes were subjected to
Earl S Ford
Chest, 147(4), 989-998 (2014-11-07)
Numbers and rates of hospitalizations and ED visits by patients with COPD are important metrics for surveillance purposes. The objective of this study was to examine trends in these rates from 2001 to 2012 among adults aged ≥ 18 years
Taichi Kano et al.
Journal of the American Chemical Society, 130(12), 3728-3729 (2008-03-07)
A direct asymmetric iodination reaction of aldehydes with NIS was found to be catalyzed by the novel axially chiral bifunctional amino alcohol (S)-1d. This method represents the rare example of the catalytic and highly enantioselective synthesis of optically active alpha-iodoaldehydes.
Cong-Ying Zhou et al.
Organic letters, 12(9), 2104-2107 (2010-04-15)
Iodination of arene-containing natural products employing N-iodosuccinimide catalyzed by In(OTf)(3) at ambient temperature is reported as a versatile and mild method for natural product derivatization amenable to small scale. This process facilitates natural product derivatization of arene moieties for SAR
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)