549835
2,4,6-Tris(dimethylamino)-1,3,5-triazine
96%
Synonym(s):
Altretamine, Hexamethylmelamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H18N6
CAS Number:
Molecular Weight:
210.28
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
mp
171-175 °C (lit.)
functional group
amine
SMILES string
CN(C)c1nc(nc(n1)N(C)C)N(C)C
InChI
1S/C9H18N6/c1-13(2)7-10-8(14(3)4)12-9(11-7)15(5)6/h1-6H3
InChI key
UUVWYPNAQBNQJQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,4,6-Tris(dimethylamino)-1,3,5-triazine exhibits antitumor activity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Triazines and related products. Part 26. Synthesis and chemistry of bicyclic analogues of the antitumour drug 2, 4, 6-tris (dimethylamino)-1, 3, 5-triazine (hexamethylmelamine).
Langdon SP, et al.
Journal of the Chemical Society. Perkin Transactions 1, 993-998 (1984)
Maurie Markman et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 24(9), 1454-1458 (2006-03-22)
A previous report suggested the nadir serum CA-125 level within the group of patients with ovarian cancer who achieved normalization of CA-125 accurately defined the risk of relapse. Using similar CA-125 subgroups, we sought to determine if the baseline CA-125
Medication sheets for patients. Oral chemotherapy.
Clinical journal of oncology nursing, 7(6 Suppl), 40-72 (2004-01-07)
Nina Keldsen et al.
Gynecologic oncology, 88(2), 118-122 (2003-02-15)
To evaluate the activity of oral Altretamine in women with epithelial ovarian carcinoma who responded (PR or CR) to first line chemotherapy but relapsed within 6 months. The protocol was later amended to include patients with relapse within 12 months.
R T Zon et al.
Investigational new drugs, 19(3), 229-231 (2001-09-20)
Thirty patients with advanced renal cell carcinoma were treated on a phase 11 trial with altretamine. Altretamine was administered orally at a dosage of 260 mg/m2 days 1 through 14 with cycles repeated every 28 days. Nausea and vomiting were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service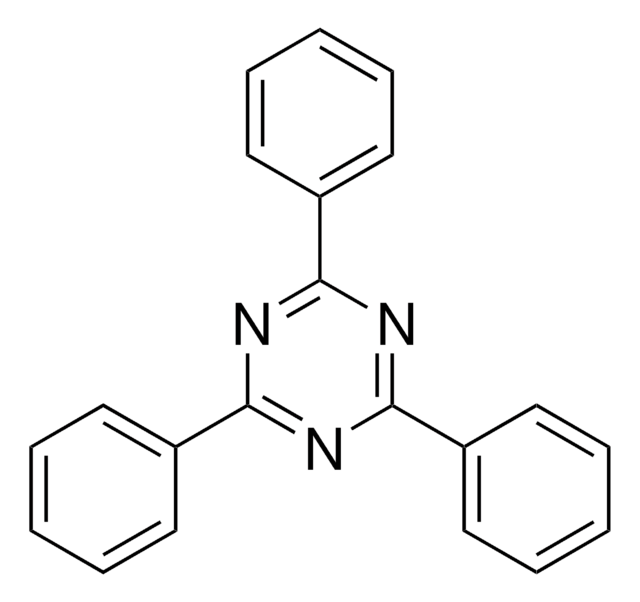



![Bis[2-(methacryloyloxy)ethyl] phosphate](/deepweb/assets/sigmaaldrich/product/structures/128/336/4e7a3e38-338c-423e-95b8-70d9d1f8e121/640/4e7a3e38-338c-423e-95b8-70d9d1f8e121.png)