All Photos(1)
About This Item
Empirical Formula (Hill Notation):
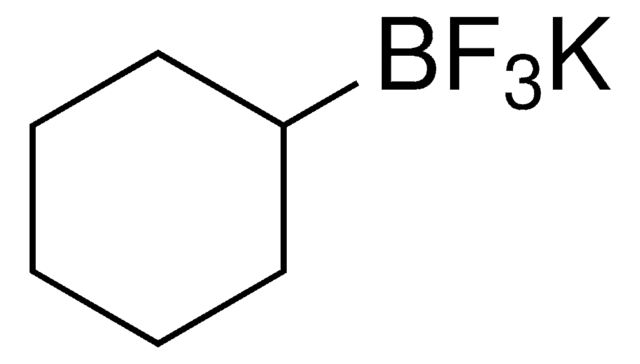
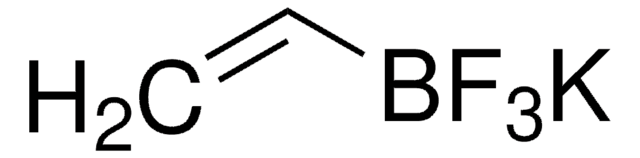
C3H5BF3K
CAS Number:
Molecular Weight:
147.98
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
powder
mp
348-350 °C
SMILES string
[K+].F[B-](F)(F)C1CC1
InChI
1S/C3H5BF3.K/c5-4(6,7)3-1-2-3;/h3H,1-2H2;/q-1;+1
InChI key
CFMLURFHOSOXRC-UHFFFAOYSA-N
Related Categories
General description
May contain 5-10% cyclopropylboronic acid
Application
Organotrifluoroborate involved in Suzuki-Miyaura cross-coupling reactions
Organotrifluoroborates as versatile and stable boronic acid surrogates.
Organotrifluoroborates as versatile and stable boronic acid surrogates.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Guo-Hua Fang et al.
Organic letters, 6(3), 357-360 (2004-01-30)
[reaction: see text] Stereospecific cyclopropanation of alkenylboronic esters of pinacol followed by in situ treatment with excess KHF(2) afforded the corresponding potassium cyclopropyl trifluoroborates in high yields, which then underwent Suzuki-Miyaura cross-coupling reactions with aryl bromides to give cyclopropyl-substituted arenes
Charette, A. B. et al.
Synlett, 1779-1779 (2005)
Articles
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service