C112909
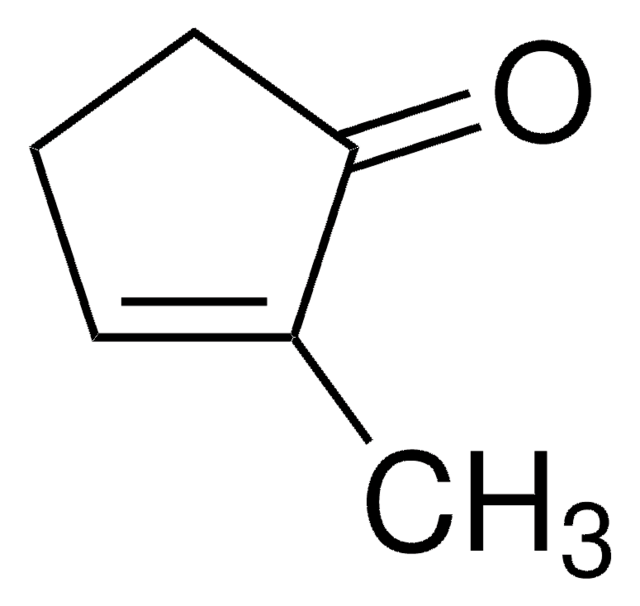
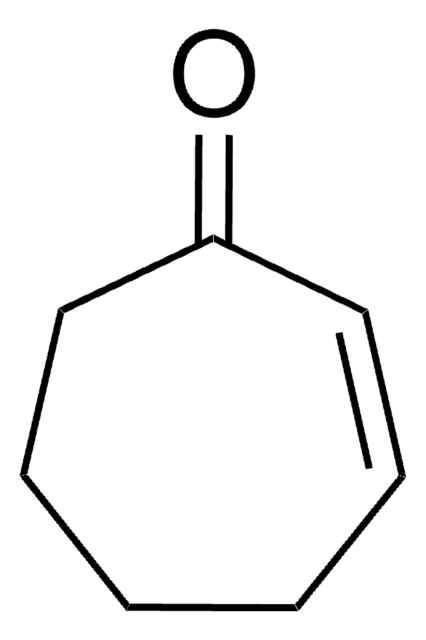
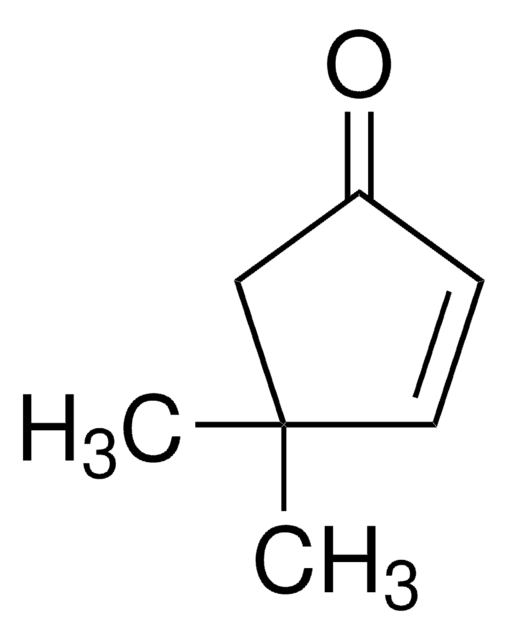
2-Cyclopenten-1-one
98%
Synonym(s):
1-Cyclopenten-3-one, 1-Cyclopenten-5-one, 2-Cyclopentenone, Cyclopent-2-en-1-one, Cyclopenten-3-one
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H6O
CAS Number:
Molecular Weight:
82.10
Beilstein:
1446054
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.481 (lit.)
bp
64-65 °C/19 mmHg (lit.)
density
0.98 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
O=C1CCC=C1
InChI
1S/C5H6O/c6-5-3-1-2-4-5/h1,3H,2,4H2
InChI key
BZKFMUIJRXWWQK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Versatile electrophile employed in a variety of addition reactions including conjugate addition of organocopper nucleophiles, Michael reaction with silyl enol ethers, and siloxanes, Diels-Alder cycloadditions, and phosphoniosilylations.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
107.6 °F - closed cup
Flash Point(C)
42 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Filippo De Simone et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(51), 14527-14538 (2011-11-25)
The Nazarov cyclization of divinyl ketones gives access to cyclopentenones. Replacing one of the vinyl groups by a cyclopropane leads to a formal homo-Nazarov process for the synthesis of cyclohexenones. In contrast to the Nazarov reaction, the cyclization of vinyl-cyclopropyl
Tetrahedron Letters, 34, 6777-6777 (1993)
Jesse R Poganik et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 33(12), 14636-14652 (2019-11-02)
The nuclear factor erythroid 2-related factor 2 (Nrf2) signaling axis is a target of covalent drugs and bioactive native electrophiles. However, much of our understanding of Nrf2 regulation has been focused at the protein level. Here we report a post-transcriptional
Marc Revés et al.
Organic letters, 14(13), 3534-3537 (2012-06-28)
1,2,3,4-Tetramethyl-bicyclo[2.2.1]hepta-2,5-diene (TMNBD, for tetramethylnorbornadiene) has been prepared and used successfully as an acetylene equivalent in the synthesis of substituted cyclopentenones. TMNBD is easily accessible on a multigram scale and displays excellent reactivity toward the intermolecular Pauson-Khand reaction. Conjugate additions on
Yoshihiro Sumiyoshi et al.
The Journal of chemical physics, 125(12), 124307-124307 (2006-10-04)
Pure rotational transitions in the ground state for Ar-OH and Ar-OD [Y. Ohshima et al., J. Chem. Phys. 95, 7001 (1991) and Y. Endo et al., Faraday Discuss. 97, 341 (1994)], those in the excited states of the OH vibration
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service