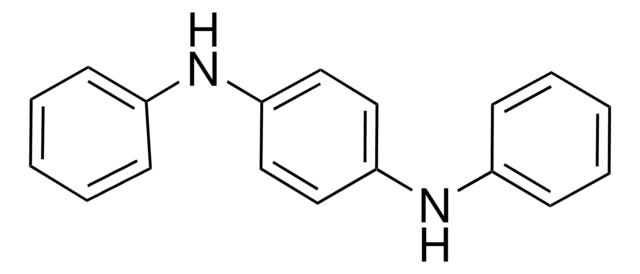
D27004
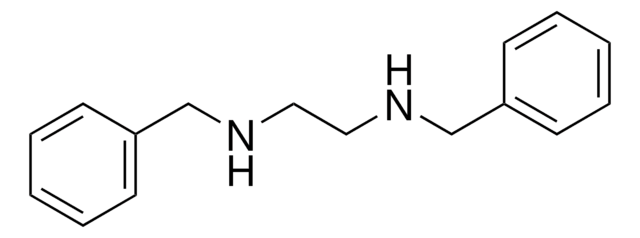
N,N′-Diphenylethylenediamine
98%
Synonym(s):
1,2-Dianilinoethane, N,N′-Ethylenedianiline, Wanzlick’s Reagent for aldehydes
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5NHCH2CH2NHC6H5
CAS Number:
Molecular Weight:
212.29
Beilstein:
646740
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
65-67 °C (lit.)
SMILES string
C(CNc1ccccc1)Nc2ccccc2
InChI
1S/C14H16N2/c1-3-7-13(8-4-1)15-11-12-16-14-9-5-2-6-10-14/h1-10,15-16H,11-12H2
InChI key
NOUUUQMKVOUUNR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N,N′-Diphenylethylenediamine can be used:
- To prepare nickel(II) chelates to study their chemical reactivities.
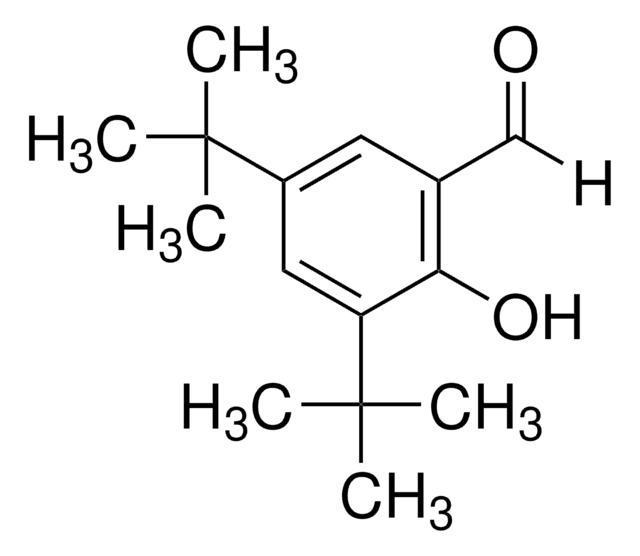
- To prepare N-heterocyclic carbene (NHC) adducts by reacting with substituted benzaldehydes.
- As a starting material to prepare substituted cyclic poly(methyl methacrylate)s.
Other Notes
Remainder mainly 1,4-diphenylpiperazine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J. Korean Chem. Soc., 36, 872-872 (1992)
Takashi Yoshitake et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 807(2), 177-183 (2004-06-19)
A highly selective and sensitive column liquid chromatographic method for fluorescence determination of serotonin (5-HT), dopamine (DA), noradrenaline (NA) and their related metabolites 5-hydroxyindole-3-acetic acid (5-HIAA) and 3,4-dihydroxyphenylacetic acid (DOPAC) following derivatization with benzylamine and 1,2-diphenylethylenediamine (DPE) is described. The
A general and versatile approach to thermally generated N-heterocyclic carbenes
Nyce GW, et al.
Chemistry?A European Journal , 10(16), 4073-4079 (2004)
Effect of electronic structure on the photoinduced ligand exchange of Ru(II) polypyridine complexes.
Robert N Garner et al.
Inorganic chemistry, 50(10), 4384-4391 (2011-04-21)
The series of complexes [Ru(bpy)(2)(L)](2+), where bpy = 2,2'-bipyridine and L = 3,6-dithiaoctane (bete, 1), 1,2-bis(phenylthio)ethane (bpte, 2), ethylenediamine (en, 3), and 1,2-dianilinoethane (dae, 4), were synthesized, and their photochemistry was investigated. Photolysis experiments show that the bisthioether ligands in
Chemische Berichte, 86, 1463-1463 (1953)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,2,4]Triazolo[1,5-a][1,3,5]triazin-7-amine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/362/413/8a902135-3f29-47f0-8393-a194caf2c230/640/8a902135-3f29-47f0-8393-a194caf2c230.png)