V209
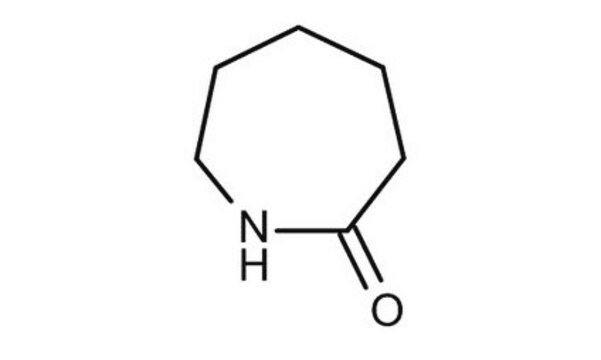
δ-Valerolactam
98%
Synonym(s):
delta-Valerolactam, 2-Piperidone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H9NO
CAS Number:
Molecular Weight:
99.13
Beilstein:
106434
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
256 °C (lit.)
81-82 °C/0.1 mmHg (lit.)
mp
38-40 °C (lit.)
SMILES string
O=C1CCCCN1
InChI
1S/C5H9NO/c7-5-3-1-2-4-6-5/h1-4H2,(H,6,7)
InChI key
XUWHAWMETYGRKB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Haili Lin et al.
Experimental & molecular medicine, 52(3), 367-379 (2020-03-11)
The function of the fibrinolytic system was first identified to dissolve fibrin to maintain vascular patency. Connections between the fibrinolytic system and many other physiological and pathological processes have been well established. Dysregulation of the fibrinolytic system is closely associated
Mitchell G Thompson et al.
ACS synthetic biology, 9(1), 53-62 (2019-12-17)
Caprolactam is an important polymer precursor to nylon traditionally derived from petroleum and produced on a scale of 5 million tons per year. Current biological pathways for the production of caprolactam are inefficient with titers not exceeding 2 mg/L, necessitating
Asymmetric synthesis of gamma-keto-delta-lactam derivatives: application to the synthesis of a conformationally constrained surrogate of Ala-Ser dipeptide.
S D Koulocheri et al.
The Journal of organic chemistry, 66(23), 7915-7918 (2001-11-10)
S Gordon et al.
Farmaco (Societa chimica italiana : 1989), 52(10), 603-608 (1998-05-15)
The synthesis of a series of 2-amino-4-hydroxy-delta-valerolactam derivatives is described (compounds 4 to 10). These compounds showed a high anthelmintic in vitro activity against the Nippostrongylus brasiliensis model.
Hiroshi Tsuchikawa et al.
Organic letters, 14(9), 2326-2329 (2012-04-26)
Chiral 2-piperidinone compounds with various C-6 substituents were successfully synthesized via a Pd-catalyzed asymmetric 6-endo cyclization of dienamides, which were evidently activated by both N-p-toluenesulfonyl and C-3 ester substituents.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service