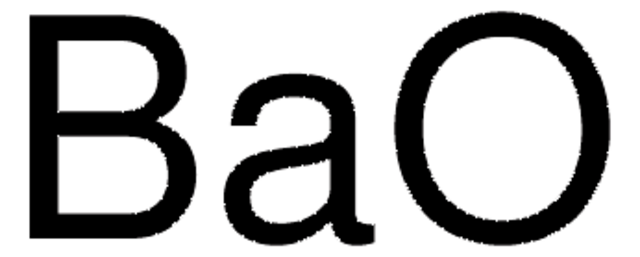202711
Barium carbonate
99.999% trace metals basis
Synonym(s):
Barium monocarbonate, Witherite
About This Item
Recommended Products
Quality Level
Assay
99.999% trace metals basis
form
powder and chunks
impurities
≤15.0 ppm Trace Metal Analysis
SMILES string
[Ba++].[O-]C([O-])=O
InChI
1S/CH2O3.Ba/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2
InChI key
AYJRCSIUFZENHW-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
- A precursor for the preparation of BaTiO3 nanoparticles, a dielectric material which is useful for manufacturing multilayer ceramic capacitors.
- A precursor for the synthesis of barium titanate thin films, which finds applications in photovoltaic devices.
Barium carbonate can also be used in following applications:
- BaCO3 nanoparticles have been reported as catalysts for high-temperature oxygen reduction reactions (ORR) in solid oxide fuel cells (SOFCs).
- Barium carbonate-based nanomaterials have been employed for sensor and catalysis applications, with improved sensing performance using one-dimensional nanostructures.
- BaCO3 is used to make barium titanate for Multi-layer ceramic capacitor and composite oxides, as well as for PTC thermistors. It′s also used to make optical glass, sputter glass for semiconductors, and as a component of phosphor materials.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PDSCL
Deleterious substance
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
202711-25G:4548173113777
202711-6X25G:4548173113791
202711-30G:
202711-VAR:
202711-5G:4548173113784
202711-6X100G:4548173250489
202711-BULK:
202711-100G:4548173113760
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Three approaches generate white light, including LED-based down-conversion for broader applications.
Lanthanide ions in spectral conversion enhance solar cell efficiency via photon conversion.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service