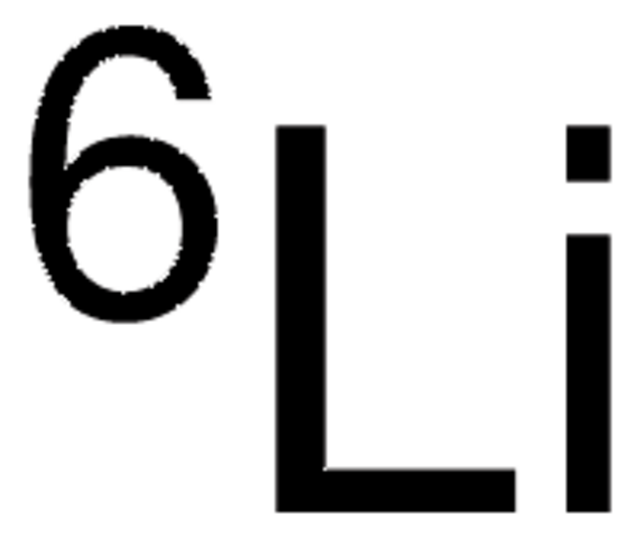278327
Lithium
wire, diam. 3.2 mm, in mineral oil, ≥98%
About This Item
Recommended Products
Assay
≥98%
form
wire
contains
copper as stabilizer
reaction suitability
reagent type: reductant
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
resistivity
9.446 μΩ-cm, 20°C
diam.
3.2 mm
impurities
0.5-1% sodium
bp
1342 °C (lit.)
mp
180 °C (lit.)
density
0.534 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
greener alternative category
SMILES string
[Li]
InChI
1S/Li
InChI key
WHXSMMKQMYFTQS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B - Water-react 1
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Group 3: Spontaneously combustible substances and water- reactive materials
Alkali metals (except potassium and sodium) and alkaline earth metals
Hazardous rank I
1st spontaneously combustible materials and water reactive materials
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
278327-BULK:
278327-100G:
278327-25G:
278327-VAR:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Nanomaterials for Energy Storage in Lithium-ion Battery Applications
HEVs address rising fuel costs and emissions concerns, utilizing battery packs alongside internal combustion engines for enhanced performance.
Professor Qiao's review explores stable microstructures for lithium metal fluoride batteries, advancing energy storage technologies.
Recent demand for electric and hybrid vehicles, coupled with a reduction in prices, has caused lithium-ion batteries (LIBs) to become an increasingly popular form of rechargeable battery technology.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service