663107
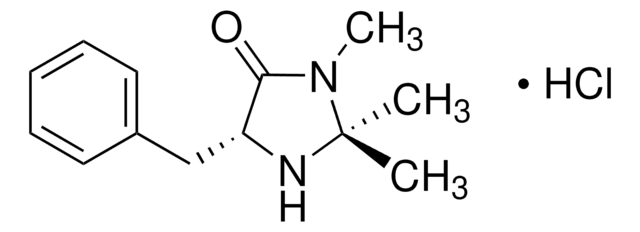
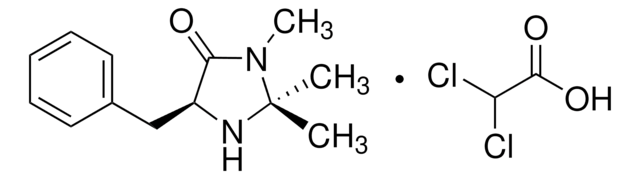
(2S,5S)-(−)-2-tert-Butyl-3-methyl-5-benzyl-4-imidazolidinone
97%
Synonym(s):
(2S,5S)-2-tert-Butyl-3-methyl-5-phenylmethyl-4-imidazolidinone, (2S,5S)-5-Benzyl-2-tert-butyl-3-methyl-4-imidazolidinone
About This Item
Recommended Products
Assay
97%
form
solid
mp
93-100 °C (lit.)
functional group
phenyl
SMILES string
CN1[C@H](N[C@@H](Cc2ccccc2)C1=O)C(C)(C)C
InChI
1S/C15H22N2O/c1-15(2,3)14-16-12(13(18)17(14)4)10-11-8-6-5-7-9-11/h5-9,12,14,16H,10H2,1-4H3/t12-,14-/m0/s1
InChI key
SKHPYKHVYFTIOI-JSGCOSHPSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- The chiral transformation reaction, including Friedel-Crafts and Mukaiyama-Michael reactions.
- The preparation of substituted spiroundecenetriones via asymmetric domino Knoevenagel/Diels-Alder reactions.
- The asymmetric synthesis of β-hydroxy aldehydes and their dimethylacetals via aldehyde-aldehyde aldol condensation reaction.
- The enantioselective α-fluorination of aldehydes using N-fluorobenzenesulfonamide as a fluorinating agent.
- The stereoselective preparation of (oxomethyl)oxabicyclo[3.2.1]octenones and tricyclic pyrroles via [4+3] cycloaddition of (trialkylsiloxy)pentadienals to furans.
Features and Benefits
- Superior enantiocontrol in numerous transformations
- High activities at low catalyst loadings
- Extraordinary functional group tolerance
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
663107-BULK:
663107-VAR:
663107-500MG:
663107-1G:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Discover Professor David MacMillan's groundbreaking metal-free asymmetric catalysis using imidazolidinone-based organocatalysts for versatile transformations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether 97%](/deepweb/assets/sigmaaldrich/product/structures/396/398/09a397b1-b5f5-420f-98da-adf9017cef56/640/09a397b1-b5f5-420f-98da-adf9017cef56.png)


