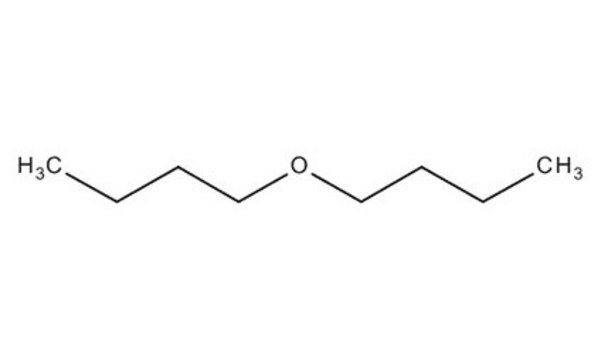
110280
Dibutyl ether
ReagentPlus®, ≥99%
Synonym(s):
Butyl ether
About This Item
Recommended Products
vapor density
4.48 (vs air)
Quality Level
vapor pressure
4.8 mmHg ( 20 °C)
product line
ReagentPlus®
Assay
≥99%
form
liquid
autoignition temp.
365 °F
expl. lim.
8.5 %
refractive index
n20/D 1.399 (lit.)
pH
5.2
bp
142-143 °C (lit.)
mp
−98 °C (lit.)
density
0.764 g/mL at 25 °C (lit.)
SMILES string
CCCCOCCCC
InChI
1S/C8H18O/c1-3-5-7-9-8-6-4-2/h3-8H2,1-2H3
InChI key
DURPTKYDGMDSBL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- To evaluate the anthracene solubility in various binary solvent mixtures composed of dibutyl ether along with n-hexane, cyclohexane, n-heptane, methylcyclohexane, n-octane, isooctane and cyclooctane.
- To measure the pyrene solubility in various binary solvent mixtures composed of dibutyl ether along with n-hexane, cyclohexane, n-heptane, methylcyclohexane, n-octane, isooctane and tert-butylcyclohexane.
- To investigate the reaction mechanism and kinetic studies of the reaction of phenyl isocyanate with methanol in the presence of dibutyltin diacetate (catalyst) to afford urethane. Rate constant of the reaction, k was reported to be 0.96liter/(mole sec).
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
82.4 °F - closed cup
Flash Point(C)
28 °C - closed cup
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Group 4: Flammable liquids
Type 2 petroleums
Hazardous rank III
Water insoluble liquid
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
110280-500ML:
110280-2L:
110280-2.5L:
110280-VAR:
110280-200L:
110280-200L-PW:
110280-18L-CS:
110280-20L:
110280-VAR-D:
110280-1L:
110280-BULK:
110280-18L:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service