103012
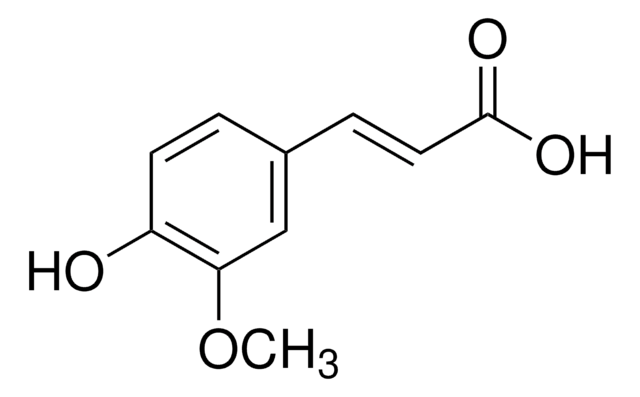
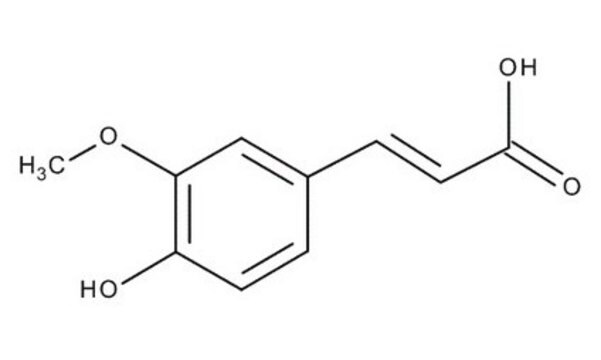
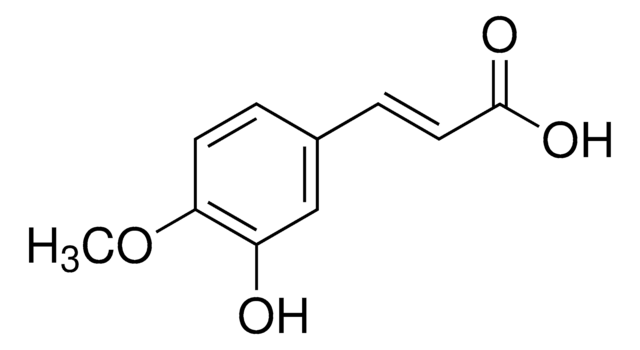
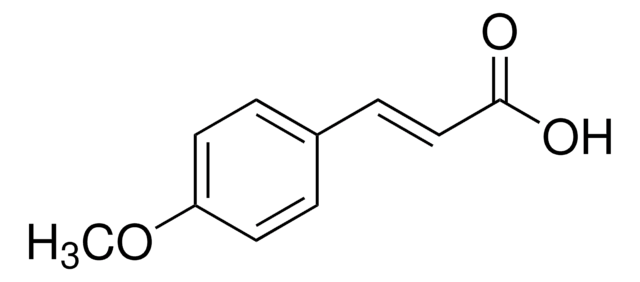
3-Hydroxy-4-methoxycinnamic acid, predominantly trans
97%
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HOC6H3(OCH3)CH=CHCO2H
CAS Number:
Molecular Weight:
194.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
230 °C (dec.) (lit.)
functional group
carboxylic acid
SMILES string
COc1ccc(\C=C\C(O)=O)cc1O
InChI
1S/C10H10O4/c1-14-9-4-2-7(6-8(9)11)3-5-10(12)13/h2-6,11H,1H3,(H,12,13)/b5-3+
InChI key
QURCVMIEKCOAJU-HWKANZROSA-N
Related Categories
General description
3-Hydroxy-4-methoxycinnamic acid, predominantly trans is available as white crystals. 3-Hydroxy-4-methoxycinnamic acid is isolated from the aerial part of Artemisia capillaris, Chinese medicinal plant. It is a component of chinese herbal medicine used for a pain killer and stomachic. It is an efficient acetylcholine inhibitor. 3-Hydroxy-4-methoxycinnamic acid is bioactive metabolite of Spilanthes acmella Murr. It increases the resistance of low-density lipoprotein (LDL) to oxidation.
Application
3-Hydroxy-4-methoxycinnamic acid was used in the synthesis of tranilast and various tranilast analogs (cinnamoyl anthranilates) by genetically engineered Saccharomyces cerevisiae yeast strain. It was also used in the synthesis of glycoside compounds by undergoing glycosidation.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T S Wu et al.
Bioorganic & medicinal chemistry, 9(1), 77-83 (2001-02-24)
Five new constituents including a flavonoid, artemisidin A (1), and four coumarins, artemicapins A (2), B (3), C (4) and D (5), together with 70 known compounds (6-75), have been isolated and characterized from the aerial part of Artemisia capillaris.
Mathieu Renouf et al.
The Journal of nutrition, 140(2), 259-263 (2009-12-17)
Chlorogenic acids (CGA) are antioxidants found in coffee. They are becoming of interest for their health-promoting effects, but bioavailability in humans is not well understood. We hypothesized that adding whole milk or sugar and nondairy creamer to instant coffee might
I-Min Liu et al.
The Journal of pharmacology and experimental therapeutics, 307(3), 1196-1204 (2003-09-17)
We investigated the mechanism(s) by which isoferulic acid lowers plasma glucose levels in streptozotocin-induced diabetic rats (STZ-diabetic rats). In STZ-diabetic rats, isoferulic acid dose dependently lowered plasma glucose concentrations and increased plasma beta-endorphin-like immunoreactivity (BER). Both of these effects of
Supaluk Prachayasittikul et al.
Molecules (Basel, Switzerland), 14(2), 850-867 (2009-03-04)
Spilanthes acmella Murr. (Compositae) has been used as a traditional medicine for toothache, rheumatism and fever. Its extracts had been shown to exhibit vasorelaxant and antioxidant activities. Herein, its antimicrobial, antioxidant and cytotoxic activities were evaluated. Agar dilution method assays
Adriana Farah et al.
The Journal of nutrition, 138(12), 2309-2315 (2008-11-22)
Chlorogenic acids (CGA) are cinnamic acid derivatives with biological effects mostly related to their antioxidant and antiinflammatory activities. Caffeoylquinic acids (CQA) and dicaffeoylquinic acids (diCQA) are the main CGA found in nature. Because green coffee is a major source of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service