11715
Sodium barbiturate
≥97.0% (T)
Synonym(s):
Barbituric acid sodium salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H3N2NaO3
CAS Number:
Molecular Weight:
150.07
Beilstein:
3748030
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (T)
solubility
hot water: soluble 0.2 g/10 mL, clear, colorless
SMILES string
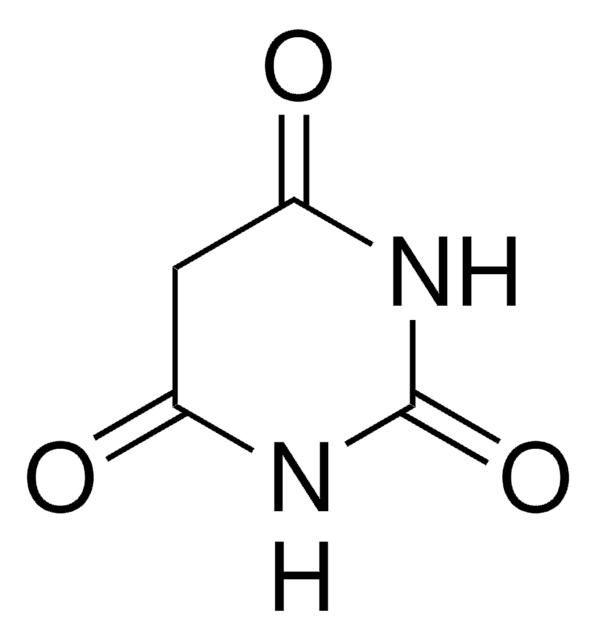
[Na+].[O-]C1=NC(=O)NC(=O)C1
InChI
1S/C4H4N2O3.Na/c7-2-1-3(8)6-4(9)5-2;/h1H2,(H2,5,6,7,8,9);/q;+1/p-1
InChI key
MHQHHBYRYFICDV-UHFFFAOYSA-M
General description
Sodium barbiturate is a white to light beige powder. It accelerates the propagation of metastatic prostate adenocarcinomas in rats and in tissue culture.
Application
Sodium barbiturate has been used for quantification of thrombin in plasma. It has been used in the estimation of trypsin in duodenal contents. It was also used as a buffer to characterise the functions of two long-chain fatty acid CoA ligase genes (facl) in crude oil-degrading Geobacillus thermodenitrificans NG80-2.
Other Notes
Sales restrictions may apply
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Pollard et al.
Oncology, 34(3), 129-132 (1977-01-01)
Metastatic prostate adenocarcinomas, derived from aging germfree Wistar rats, have been propagated in rats and in tissue culture. A protocol has been developed and demonstrated for assay of treatments which retard or which accelerate the rate and extent of tumor
F A Ofosu et al.
Blood, 64(3), 742-747 (1984-09-01)
Heparan with a low affinity for antithrombin III has previously been demonstrated to inhibit thrombin generation in both normal plasma and plasma depleted of antithrombin III. In addition, standard heparin and heparin with a low affinity for antithrombin III have
Simple method for estimating trypsin.
H S Wiggins
Gut, 8(4), 415-416 (1967-08-01)
Vadivel Parthsarathy et al.
PloS one, 8(1), e54769-e54769 (2013-02-06)
Previously, we have developed a retro-inverso peptide inhibitor (RI-OR2, rGffvlkGr) that blocks the in vitro formation and toxicity of the Aβ oligomers which are thought to be a cause of neurodegeneration and memory loss in Alzheimer's disease. We have now
Szilvia Hajdok et al.
The Journal of organic chemistry, 74(19), 7230-7237 (2009-09-11)
The laccase-catalyzed reaction between catechols and heterocyclic 1,3-dicarbonyls (pyridinones, quinolinones, thiocoumarins) using aerial oxygen as the oxidant delivers benzofuropyridinones, benzofuroquinolinones, and thiocoumestans in a simple fashion, highly regioselectively with yields ranging from 55 to 98%. With barbituric acid derivatives the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service