158186
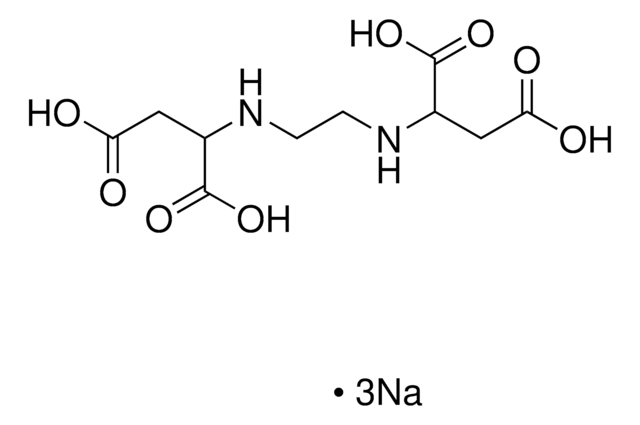
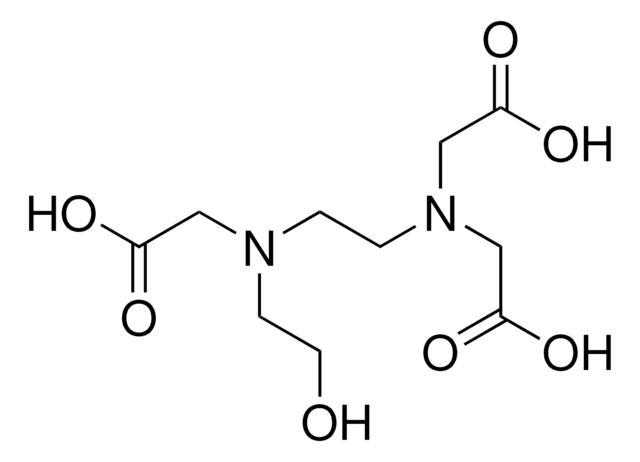
Ethylenediamine-N,N′-diacetic acid
≥98%
Synonym(s):
EDDA, N,N′-Ethylenediglycine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HOOCCH2NHCH2CH2NHCH2COOH
CAS Number:
Molecular Weight:
176.17
Beilstein:
1778355
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
mp
224 °C (dec.) (lit.)
functional group
amine
carboxylic acid
SMILES string
OC(=O)CNCCNCC(O)=O
InChI
1S/C6H12N2O4/c9-5(10)3-7-1-2-8-4-6(11)12/h7-8H,1-4H2,(H,9,10)(H,11,12)
InChI key
IFQUWYZCAGRUJN-UHFFFAOYSA-N
Application
Ethylenediamine-N,N′-diacetic acid (EDDA) is a chelating agent that can be used to synthesize:
- Binary and ternary copper(II) complexes with potent proteasome inhibitory properties.
- Pd(EDDA) complexes which can coordinate with amino acids, peptides, or DNA units.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hoi-Ling Seng et al.
Journal of inorganic biochemistry, 102(11), 1997-2011 (2008-09-10)
The binding selectivity of the M(phen)(edda) (M=Cu, Co, Ni, Zn; phen=1,10-phenanthroline, edda=ethylenediaminediacetic acid) complexes towards ds(CG)(6), ds(AT)(6) and ds(CGCGAATTCGCG) B-form oligonucleotide duplexes were studied by CD spectroscopy and molecular modeling. The binding mode is intercalation and there is selectivity towards
Olivier Dalmas et al.
Structure (London, England : 1993), 18(7), 868-878 (2010-07-20)
The transmembrane conformation of Thermotoga maritima CorA, a magnesium transport system, has been studied in its Mg(2+)-bound form by site-directed spin labeling and electron paramagnetic resonance spectroscopy. Probe mobility together with accessibility data were used to evaluate the overall dynamics
Giuseppe Palladino et al.
Dalton transactions (Cambridge, England : 2003), (43)(43), 5176-5183 (2006-11-02)
The structure, thermodynamics and kinetics of the binary and ternary uranium(VI)-ethylenediamine-N,N'-diacetate (in the following denoted EDDA) fluoride systems have been studied using potentiometry, 1H, 19F NMR spectroscopy and X-ray diffraction. The UO2(2+)-EDDA system could be studied up to -log[H3O+] =
Karla Mettrick et al.
Antibiotics (Basel, Switzerland), 9(4) (2020-04-02)
Targeting the iron requirement of Pseudomonas aeruginosa may be an effective adjunctive for conventional antibiotic treatment against biofilm-dwelling P. aeruginosa. We, therefore, assessed the anti-biofilm activity of N,N'-bis (2-hydroxybenzyl) ethylenediamine-N,N'-diacetic acid (HBED), which is a synthetic hexadentate iron chelator. The
Clara L Santos-Cuevas et al.
Nuclear medicine communications, 29(8), 741-747 (2008-08-30)
The gastrin-releasing peptide receptor (GRP-R) is expressed in several normal human tissues and is overexpressed in various human tumors including breast, prostate, small-cell lung cancer and pancreatic cancer. Recently, 99mTc-EDDA/HYNIC-[Lys]-bombesin (99mTc-HYNIC-BN) was reported as a radiopharmaceutical with high stability in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service