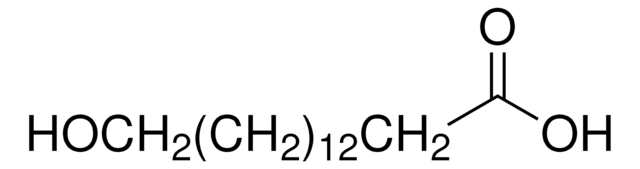177490
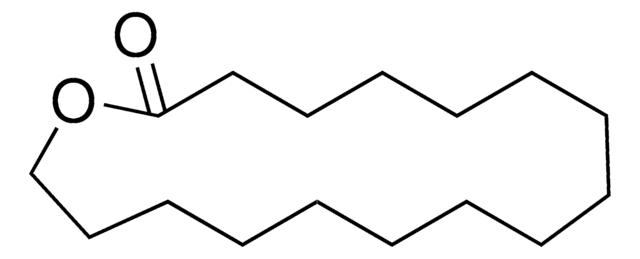
16-Hydroxyhexadecanoic acid
98%
Synonym(s):
16-Hydroxypalmitic acid, Juniperic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HO(CH2)15CO2H
CAS Number:
Molecular Weight:
272.42
Beilstein:
1783998
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
94-98 °C (lit.)
functional group
carboxylic acid
hydroxyl
SMILES string
OCCCCCCCCCCCCCCCC(O)=O
InChI
1S/C16H32O3/c17-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16(18)19/h17H,1-15H2,(H,18,19)
InChI key
UGAGPNKCDRTDHP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
16-Hydroxyhexadecanoic acid was used in the synthesis of dihydroxypalmitic acids. It was also used to induce the expression of two GRP genes of Arabidopsis thaliana, AtGRP5 and AtGRP23.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B Grausem et al.
Plant, cell & environment, 37(9), 2102-2115 (2014-02-14)
Cutin and suberin represent lipophilic polymers forming plant/environment interfaces in leaves and roots. Despite recent progress in Arabidopsis, there is still a lack on information concerning cutin and suberin synthesis, especially in crops. Based on sequence homology, we isolated two
The importance of water to biocatalysis in organic solvents.
M J Alston et al.
Biochemical Society transactions, 23(1), 70S-70S (1995-02-01)
Peiyao Zhu et al.
Journal of cell communication and signaling, 14(2), 175-192 (2020-01-12)
Esophageal squamous cell carcinoma (ESCC) is one of the most common malignant tumors with poor prognosis. Aryl hydrocarbon receptor (AHR) is a ligand-dependent transcription factor and emerging evidence shows it is associated with tumor initiation and promotion. However, the relationship
Nejumal K Khalid et al.
Environmental monitoring and assessment, 190(6), 370-370 (2018-06-02)
The presence of emerging contaminants (ECs) in different aquatic systems may contribute to hazardous effects on aquatic organisms and subsequently on human health. In the present work, liquid chromatography coupled to a quadrupole time of flight mass spectrometer (LC-Q-ToF-MS) was
Sapa Hima Rani et al.
The Journal of biological chemistry, 285(49), 38337-38347 (2010-10-06)
A key step in the triacylglycerol (TAG) biosynthetic pathway is the final acylation of diacylglycerol (DAG) by DAG acyltransferase. In silico analysis has revealed that the DCR (defective in cuticular ridges) (At5g23940) gene has a typical HX(4)D acyltransferase motif at
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service