187305
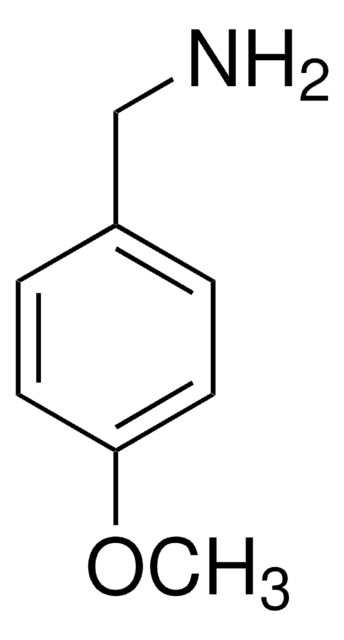
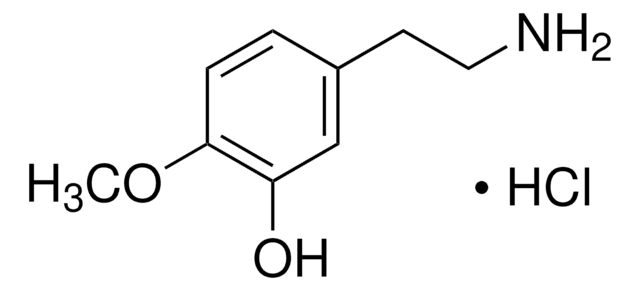
4-Methoxyphenethylamine
≥98%
Synonym(s):
2-(4-Methoxyphenyl)ethylamine, 4-Methoxyphenethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H4CH2CH2NH2
CAS Number:
Molecular Weight:
151.21
Beilstein:
508967
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
liquid
refractive index
n20/D 1.538 (lit.)
bp
138-140 °C/20 mmHg (lit.)
254-256 °C
density
1.031 g/mL at 20 °C (lit.)
functional group
amine
SMILES string
COc1ccc(CCN)cc1
InChI
1S/C9H13NO/c1-11-9-4-2-8(3-5-9)6-7-10/h2-5H,6-7,10H2,1H3
InChI key
LTPVSOCPYWDIFU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Methoxyphenethylamine inhibits the monoamine oxidase-catalyzed deamination of both tyramine and tryptamine.
4-Methoxyphenethylamine is used as a precursor for the synthesis of other organic compounds by the alkylation reaction.
4-Methoxyphenethylamine is used as a precursor for the synthesis of other organic compounds by the alkylation reaction.
Application
4-Methoxyphenethylamine was used in the synthesis of :
- pyrrolo[3,2-c]carbazole
- poly(4-methoxyphenethylamine), required for the immobilization of nitrogenated bases and oligonucleotides
- organopolyphosphazenes such as poly[bis(4-methoxy benzylamino)polyphosphazene] and poly[bis(4-methoxyphenethylamino)polyphosphazene]
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
GC--MS analysis of N-(bromodimethoxybenzyl)-2-, 3-, and 4-methoxyphenethylamines: Inverse analogues of the psychoactive 25B-NBOMe drug
Almalki AJ, et al.
Forensic Chemistry, 21, 100277-100277 (2020)
Electrochemical Investigation of oligonucleotide-DNA hybridization on poly(4-methoxyphenethylamine).
Francielle B Silva et al.
International journal of molecular sciences, 9(7), 1173-1188 (2009-03-28)
This work describes the immobilization of purine and pyrimidine bases and immobilization/hybridization of synthetic oligonucleotides on graphite electrodes modified with poly(4-methoxyphenethylamine) produced in acid medium. The immobilization of adenine, guanine, cytosine and thymine on these modified electrodes was efficient, producing
Synthesis and characterization of novel polyorganophosphazenes substituted with 4-methoxybenzylamine and 4-methoxyphenethylamine for in vitro release of indomethacin and 5-fluorouracil.
Gudasi KB, et al.
Reactive functional Polymers, 66(10), 1149-1157 (2006)
V I Kulinskiĭ et al.
Radiobiologiia, 33(1), 137-140 (1993-01-01)
Specific 2-propynylamine inhibitors of monoamine oxidase (MAO) pargyline and especially chlorgyline, a selective inhibitor of MAO A (but not deprenyl, a selective inhibitor of MAO B) increase the radioprotective effect of small doses of O-methyltyramine and phenylephrine and do not
Justin L Neill et al.
Physical chemistry chemical physics : PCCP, 13(16), 7253-7262 (2011-03-12)
Recent advances in the technology of test and measurement equipment driven by the computer and telecommunications industries have made possible the development of a new broadband, Fourier-transform microwave spectrometer that operates on principles similar to FTNMR. This technique uses a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service