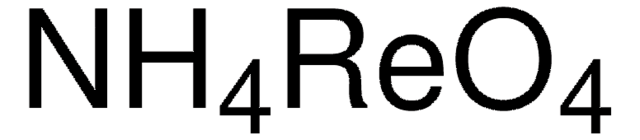All Photos(2)
About This Item
Linear Formula:
KReO4
CAS Number:
Molecular Weight:
289.30
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
form:
powder
Recommended Products
Quality Level
Assay
99.98% trace metals basis
form
powder
impurities
≤250.0 ppm Trace Metal Analysis
mp
550 °C (lit.)
density
4.887 g/mL at 25 °C (lit.)
SMILES string
[K+].[O-][Re](=O)(=O)=O
InChI
1S/K.4O.Re/q+1;;;;-1;
InChI key
QFKRWIFGDGKWLM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
The reduction of KReO4 in the preparation of cyclopentadienyltricarbonylrhenium complexes has potential as a model for the syntheses of technetium and rhenium organometallic radiopharmaceuticals.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Spradau, T.W. Katzenellenbogen, J.A.
Organometallics, 17, 2009-2009 (1998)
New sequencing technique produces high-resolution map of 5-hydroxymethylcytosine.
Jonathan Wilkinson
Epigenomics, 4(3), 249-249 (2012-08-10)
Marie Aufort et al.
Chembiochem : a European journal of chemical biology, 12(4), 583-592 (2011-02-10)
The parallel oxorhenium-mediated assembly of 288 noncyclic RGD analogues is reported. All complexes contain a NS(2) +S chelating motif that enables the unambiguous coordination of the oxorhenium and oxotechnetium cores. In this study, "modules S" contain a variety of pending
Rui Cao et al.
Inorganic chemistry, 50(19), 9499-9507 (2011-08-30)
We describe a multidentate tripodal ligand in which three pendant arms carrying di(2-picolyl)amine units are linked to the ortho positions of a tris(o-xylyl) scaffold, providing N(CH(2)-o-C(6)H(4)CH(2)N(CH(2)py)(2))(3) (L). Reaction of L with CuCl(2) in the presence of hexafluorophosphate anion afforded blue
Julie Di Bernardo et al.
Analytical biochemistry, 415(1), 32-38 (2011-05-07)
The sodium/iodide symporter (NIS) is primarily responsible for iodide accumulation in the thyroid gland for the synthesis of thyroid hormones; however, it can also transport other lyotropic anions in the thyroid gland and nonthyroid tissues. Some NIS substrates have important
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service