241768
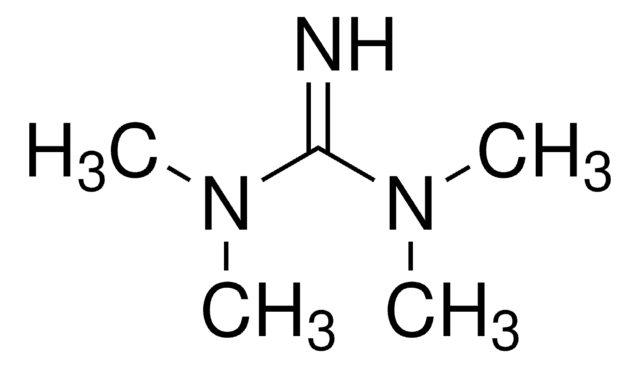
1,1,3,3-Tetramethylguanidine
99%
Synonym(s):
N,N,N′,N′-Tetramethylguanidine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2NC(=NH)N(CH3)2
CAS Number:
Molecular Weight:
115.18
Beilstein:
969608
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
0.2 mmHg ( 20 °C)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.469 (lit.)
bp
52-54 °C/11 mmHg (lit.)
density
0.918 g/mL at 25 °C (lit.)
SMILES string
CN(C)C(=N)N(C)C
InChI
1S/C5H13N3/c1-7(2)5(6)8(3)4/h6H,1-4H3
InChI key
KYVBNYUBXIEUFW-UHFFFAOYSA-N
Gene Information
human ... EPHX2(2053)
mouse ... Ephx2(13850)
Application
Base employed in the preparation of alkyl nitriles from alkyl halides and 3′-alkylthymidines from 3′-nitrothymidines.
Promotes the pentavalent bismuth oxidation of primary and secondary alchohols to aldehydes and ketones.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Met. Corr. 1 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
122.0 °F - closed cup
Flash Point(C)
50 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 48, 2885-2885 (2007)
Synthetic Communications, 23, 2323-2323 (1993)
Tetrahedron, 49, 10061-10061 (1993)
Jovana Vušurović et al.
ChemistryOpen, 6(6), 739-750 (2017-12-12)
Interactions of ribonucleic acid (RNA) with guanidine and guanidine derivatives are important features in RNA-protein and RNA-drug binding. Here we have investigated noncovalently bound complexes of an 8-nucleotide RNA and six different ligands, all of which have a guanidinium moiety
Silvia Tampucci et al.
Pharmaceutics, 12(11) (2020-11-15)
For topical treatment of skin cancer, the design of pH-responsive nanocarriers able to selectively release the drug in the tumor acidic microenvironment represents a reliable option for targeted delivery. In this context, a series of newly synthesized surface-active fatty acid-protic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)

![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)

![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)