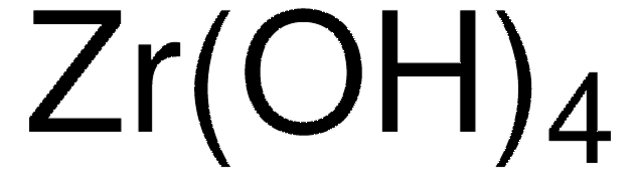
243493
Zirconium(IV) oxynitrate hydrate
99%
Synonym(s):
Zirconyl nitrate hydrate
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
ZrO(NO3)2 · xH2O
CAS Number:
Molecular Weight:
231.23 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
99%
form
powder and chunks
composition
Degree of hydration, ~6
color
white
application(s)
battery manufacturing
SMILES string
O=[Zr++].[H]O[H].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/2NO3.H2O.O.Zr/c2*2-1(3)4;;;/h;;1H2;;/q2*-1;;;+2
InChI key
BGMQNYCWALSARE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Zirconium(IV) oxynitrate hydrate is a crystalline zirconium compound that exhibits high solubility in water. It typically comprises amixture of oxide and a slightly hydroxide form.
Application
Zirconium(IV) oxynitrate hydrate is used to prepare CeO2-ZrO2 mixed oxide catalyst supports. It may be used as zirconium precursor in the preparation of;
- Pb(Zr0.50Ti0.50)O3 ferroelectric thin films,
- silica- and alumina-doped nanocrystalline ceria/zirconia (Ce0.5Zr0.5O2).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Flame-made nanocrystalline ceria/zirconia doped with alumina or silica: structural properties and enhanced oxygen exchange capacity
Schulz H, et al.
Journal of Materials Chemistry, 13(12), 2979-2984 (2003)
Influence of active metal loading and oxygen mobility on coke-free dry reforming of Ni-Co bimetallic catalysts
Djinovic P, et al.
Applied Catalysis. B, Environmental, 125, 259-270 (2012)
Sol-Gel Preparation of Pb (Zr0. 50Ti0. 50) O3 Ferroelectric Thin Films Using Zirconium Oxynitrate as the Zirconium Source
Zeng J, et al.
Journal of the American Ceramic Society. American Ceramic Society, 82(2), 461-464 (1999)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service