All Photos(1)
About This Item
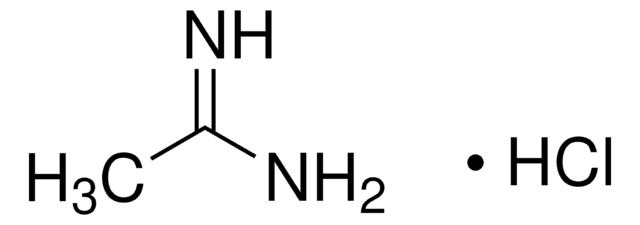
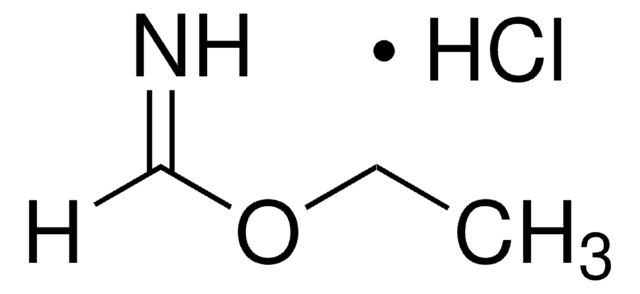
Linear Formula:
HN=CHNH2 · HCl
CAS Number:
Molecular Weight:
80.52
Beilstein:
3906935
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
84-87 °C (lit.)
functional group
amine
SMILES string
Cl[H].[H]C(N)=N
InChI
1S/CH4N2.ClH/c2-1-3;/h1H,(H3,2,3);1H
InChI key
NMVVJCLUYUWBSZ-UHFFFAOYSA-N
Application
Formamidine hydrochloride was used in the synthesis of imidazoleglycerol phosphate (IGP). It was also used in the synthesis of 5-methyl-4,6-dihydroxypyrimidine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reactions of oxidizing radicals with 4, 6-dihydroxypyrimidines as model compounds for uracil, thymine, and cytosine.
The Journal of Physical Chemistry, 91(2), 426-433 (1987)
Chung Hyeon Jang et al.
ACS nano, 14(10), 13246-13255 (2020-09-11)
A series of poly(fluorene-co-phenylene)-based anionic conjugated polyelectrolytes (CPEs) are prepared with varying sizes of counterions (tetramethylammonium, tetraethylammonium, and tetrabutylammonium (TBA+)) and studied as a hole-transporting layer (HTL) for sky-blue-emissive perovskite light-emitting diodes (PeLEDs). Ionic CPE HTLs improve the wettability, compatibility
The biosynthesis of histidine; D-erythro-imidazoleglycerol phosphate dehydrase.
B N AMES
The Journal of biological chemistry, 228(1), 131-143 (1957-09-01)
Federico Bertasi et al.
Physical chemistry chemical physics : PCCP, 19(38), 26230-26239 (2017-09-22)
This work describes the preparation of the new lipophilic ionic liquid tetraoctyl-formamidinium bis(trifluoromethanesulfonyl) imide (TOFATFSI), which is miscible with lower alkanes. In particular, this work focuses on the electric behaviour of TOFATFSI in the particularly challenging highly apolar environment of
Weiwen Zhao et al.
Organic letters, 13(19), 5160-5163 (2011-08-30)
N,N'-Disubstituted formamidines, and amidines in general, have very rich configurational, conformational, and tautomeric diversities. As part of an effort to incorporate alkoxyamine-derived formamidine units into foldamers, the first evidence for the isolation of the up-to-now unknown E isomer, the conditions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service