278564
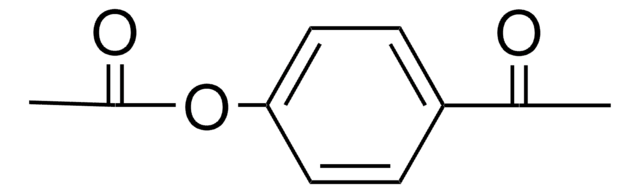
4′-Hydroxyacetophenone
99%
Synonym(s):
4-Hydroxyphenylethanone, p-Acetophenol, p-Hydroxyphenyl methyl ketone, Piceol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H4COCH3
CAS Number:
Molecular Weight:
136.15
Beilstein:
774355
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
147-148 °C/3 mmHg (lit.)
mp
109-111 °C (lit.)
solubility
95% ethanol: 5%, colorless to faintly yellow
functional group
ketone
SMILES string
CC(=O)c1ccc(O)cc1
InChI
1S/C8H8O2/c1-6(9)7-2-4-8(10)5-3-7/h2-5,10H,1H3
InChI key
TXFPEBPIARQUIG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Molecular dynamics simulation study of the two known polymorphs of 4′-hydroxyacetophenone (form I, monoclinic; form II, orthorhombic) has been described.
Application
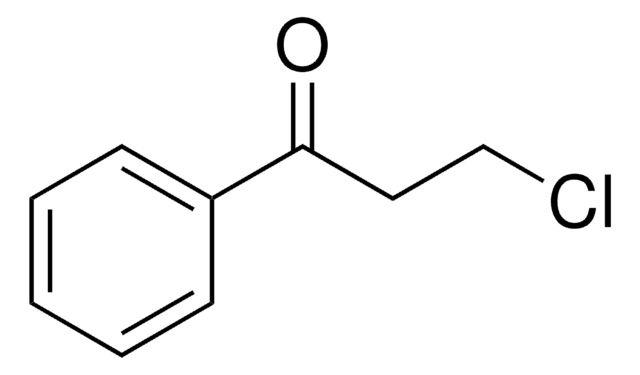
4′-Hydroxyacetophenone has been used as ketone component in the preparation of 1-aryl-3-phenethylamino-1-propanone hydrochlorides, potential cytotoxic agents, via Mannich reactions.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
330.8 °F
Flash Point(C)
166 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 1-Aryl-3-phenethylamino-1-propanone hydrochlorides as possible potent cytotoxic agents.
Ebru Mete et al.
Molecules (Basel, Switzerland), 12(12), 2579-2588 (2008-02-09)
1-Aryl-3-phenethylamino-1-propanone hydrochlorides 1-10, which are potentialpotent cytotoxic agents, were synthesized via Mannich reactions using paraformaldehyde,phenethylamine hydrochloride as the amine component and acetophenone, 4'-methyl-, 4'-methoxy-, 4'-chloro-, 4'-fluoro-, 4'-bromo-, 2',4'-dichloro-, 4'-nitro-, 4'-hydroxyacetophenone or 2-acetylthiophene as the ketone component. Yields were in the87-98
Carlos E S Bernardes et al.
The journal of physical chemistry. B, 116(17), 5179-5184 (2012-04-12)
A molecular dynamics simulation study of the two known polymorphs of 4′-hydroxyacetophenone (HAP; form I, monoclinic; form II, orthorhombic) is described. The modeling of the lattice energetics was found to be particularly sensitive to the atomic point charge (APC) selection
Colbie R Chinowsky et al.
Molecular biology of the cell, 31(25), 2803-2815 (2020-10-08)
Brush border microvilli enable functions that are critical for epithelial homeostasis, including solute uptake and host defense. However, the mechanisms that regulate the assembly and morphology of these protrusions are poorly understood. The parallel actin bundles that support microvilli have
Lars Wöhlbrand et al.
Journal of bacteriology, 190(16), 5699-5709 (2008-06-10)
The denitrifying "Aromatoleum aromaticum" strain EbN1 was demonstrated to utilize p-ethylphenol under anoxic conditions and was suggested to employ a degradation pathway which is reminiscent of known anaerobic ethylbenzene degradation in the same bacterium: initial hydroxylation of p-ethylphenol to 1-(4-hydroxyphenyl)-ethanol
Saeda Kametani et al.
Bioscience, biotechnology, and biochemistry, 71(5), 1220-1229 (2007-05-09)
The constituents of cape aloe were investigated after a preliminary screening of the growth-inhibiting effect on Ehrlich ascites tumor cells (EATC) of several extracts of this plant. Ten compounds were isolated from the dichloromethane (CH(2)Cl(2)) extract that showed the strongest
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service