All Photos(1)
About This Item
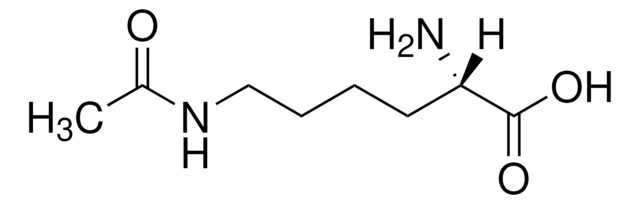
Linear Formula:
H2N(CH2)4CH(NH2)CO2H · xH2O
CAS Number:
Molecular Weight:
146.19 (anhydrous basis)
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
optical activity
[α]21/D +22°, c = 2 in 6 M HCl
reaction suitability
reaction type: solution phase peptide synthesis
application(s)
peptide synthesis
SMILES string
OC([C@@H](N)CCCCN)=O.O
InChI
1S/C6H14N2O2.H2O/c7-4-2-1-3-5(8)6(9)10;/h5H,1-4,7-8H2,(H,9,10);1H2/t5-;/m0./s1
InChI key
HZRUTVAFDWTKGD-JEDNCBNOSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dong Hoon Han et al.
Nature communications, 5, 5633-5633 (2014-12-06)
The 26S proteasome is the primary machinery that degrades ubiquitin (Ub)-conjugated proteins, including many proteotoxic proteins implicated in neurodegeneraton. It has been suggested that the elevation of proteasomal activity is tolerable to cells and may be beneficial to prevent the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service