290068
Zinc trifluoromethanesulfonate
98%
Synonym(s):
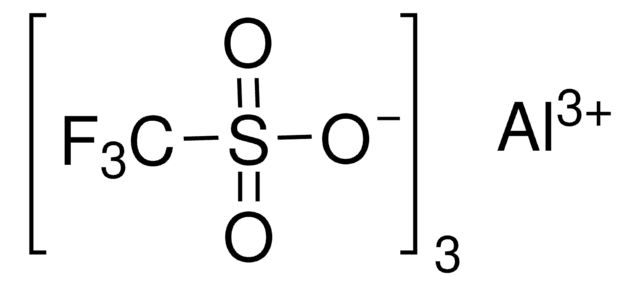
Bis(trifluoromethanesulfonato)zinc, Zinc trifluoromethylsulfonate, Zn(OTf)2, Trifluoromethanesulfonic acid zinc salt, Zinc triflate, Zn(OTf)2
About This Item
Recommended Products
Quality Level
Assay
98%
reaction suitability
core: zinc
reagent type: catalyst
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
≥300 °C (lit.)
greener alternative category
, Aligned
SMILES string
[Zn++].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/2CHF3O3S.Zn/c2*2-1(3,4)8(5,6)7;/h2*(H,5,6,7);/q;;+2/p-2
InChI key
CITILBVTAYEWKR-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Zinc trifluoromethanesulfonate acts as a lewis acid in nucleophilic addition and as a catalyst in aldol reactions.
Application
- Initiating a high-temperature zinc ion battery through a triazolium-based ionic liquid: This study explores the use of zinc trifluoromethanesulfonic acid in ionic liquid for high-temperature zinc ion batteries, demonstrating significant improvements in conductivity and stability. (Li et al., 2022).
- Enhancing the solubility of 6-mercaptopurine by formation of ionic cocrystal with zinc trifluoromethanesulfonate: The research focuses on improving the solubility of 6-mercaptopurine by forming ionic cocrystals with zinc trifluoromethanesulfonate, resulting in single-crystal-to-single-crystal transformation. (Yao et al., 2014).
- Spontaneous desaturation of the solvation sheath for high-performance anti-freezing zinc-ion gel-electrolyte: This paper discusses the development of a high-performance anti-freezing zinc-ion gel-electrolyte using zinc trifluoromethanesulfonate, which shows excellent performance even at low temperatures. (Li et al., 2023).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)