295035
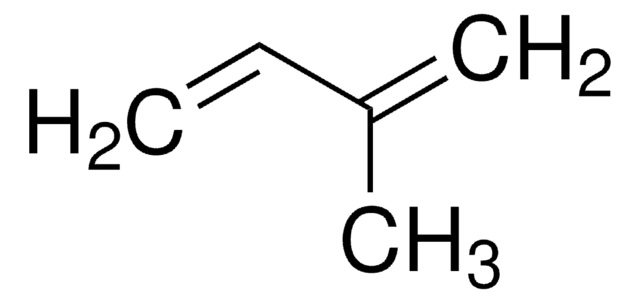
1,3-Butadiene
≥99%
Synonym(s):
Bivinyl, Vinylethylene, alpha,gamma-Butadiene
About This Item
Recommended Products
vapor density
1.9 (15 °C, vs air)
Quality Level
vapor pressure
1863 mmHg ( 21 °C)
Assay
≥99%
autoignition temp.
788 °F
contains
p-tert-butylcatechol as inhibitor
expl. lim.
12 %
bp
−4.5 °C (lit.)
mp
−109 °C (lit.)
solubility
water: soluble 0.5 g/L at 20 °C
density
0.62 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
C=CC=C
InChI
1S/C4H6/c1-3-4-2/h3-4H,1-2H2
InChI key
KAKZBPTYRLMSJV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
It may be used in the synthesis of the following:
- 1-Silyl-substituted 1,3-butadienes, by [RuHCl(CO)(PCy3)2]-catalyzed silylative coupling of terminal (E)-1,3-dienes with vinylsilanes.
- Synthetic rubber and thermoplastic resins.
- Disilylated dimers by reacting with chlorosilanes.
- Octa-2,7-dien-1-ol via palladium catalyzed-hydrodimerization.
Biochem/physiol Actions
Packaging
Compatible with the following:
Legal Information
also commonly purchased with this product
hose barb
recommended
regulator
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Gas 1A - Muta. 1B - Press. Gas Liquefied gas
Storage Class Code
2A - Gases
WGK
WGK 3
Flash Point(F)
-104.8 °F - closed cup
Flash Point(C)
-76 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service