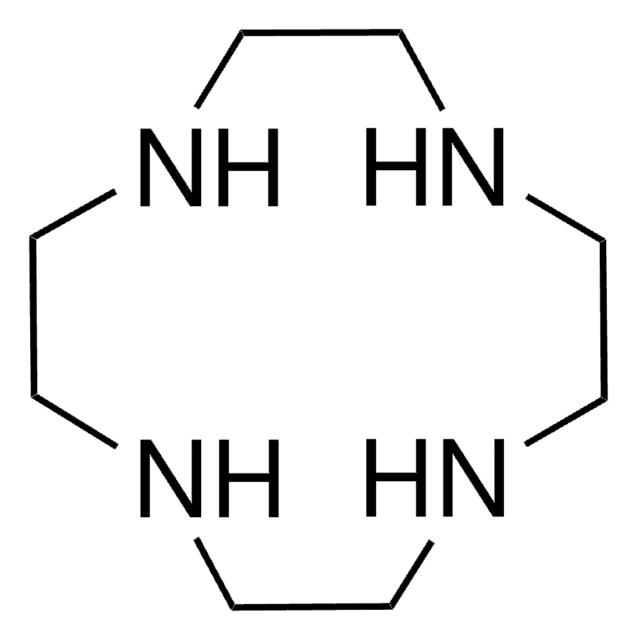
295809
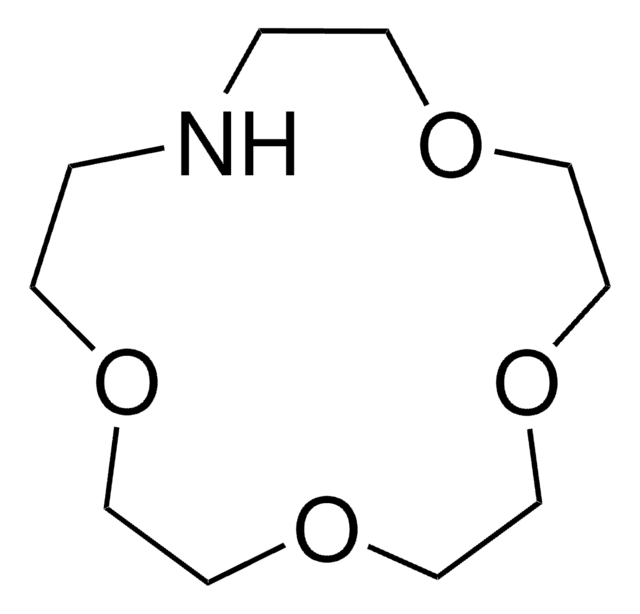
1,4,10,13-Tetraoxa-7,16-diazacyclooctadecane
≥96%
Synonym(s):
1,10-Diaza-18-crown-6
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H26N2O4
CAS Number:
Molecular Weight:
262.35
Beilstein:
609764
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥96%
form
solid
mp
111-114 °C (lit.)
functional group
ether
SMILES string
C1COCCOCCNCCOCCOCCN1
InChI
1S/C12H26N2O4/c1-5-15-9-10-17-7-3-14-4-8-18-12-11-16-6-2-13-1/h13-14H,1-12H2
InChI key
NLMDJJTUQPXZFG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,4,10,13-Tetraoxa-7,16-diazacyclooctadecane is a crown ether, which can be used:
- In the spectroscopic studies of its complex-forming reaction with iodine.
- To prepare the ligand 7,16-bis(5-t-butyl-2-hydroxybenzyl)-1,4,10,13-tetraoxa-7,16-diazacyclooctadecane.
- As a ligand in the study of zirconium facilitated hydrolysis of a dipeptide at neutral pH.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tuning Zr (IV)-assisted peptide hydrolysis at near-neutral pH
Kassai M and Grant KB
Inorganic Chemistry Communications, 11(5), 521-525 (2008)
Spectroscopic studies of the reaction of iodine with the mixed oxygen-nitrogen cyclic base 1, 4, 10, 13-tetraoxa-7, 16-diazacyclooctadecane
Nour E
Spectrochimica Acta Part A: Molecular Spectroscopy, 47(6), 743-747 (1991)
L P Varga et al.
International journal of radiation biology, 66(4), 399-405 (1994-10-01)
To date, there has been no effective therapy to counter incorporated radionuclides of strontium. In an endeavour to solve this problem, we have synthesized and evaluated various N,N'-disubstituted derivatives of 1,4,10,13-tetraoxa-7,16-diaza-cyclooctadecane(crypt and 2.2) for their ability to mobilize 85Sr2+. These
Syntheses and crystal structures of copper complexes of 7, 16-bis (5-t-butyl-2-hydroxybenzyl)-1, 4, 10, 13-tetraoxa-7, 16-diazacyclooctadecane
Ma S, et al.
Polyhedron, 22(25-26), 3249-3253 (2003)
Fran Supek et al.
European journal of medicinal chemistry, 46(8), 3444-3454 (2011-06-02)
18-crown-6 ethers are known to exert their biological activity by transporting K(+) ions across cell membranes. Using non-linear Support Vector Machines regression, we searched for structural features that influence antiproliferative activity in a diverse set of 19 known oxa-, monoaza-
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane 98%](/deepweb/assets/sigmaaldrich/product/structures/189/812/8a6555e5-8de6-4236-865f-19339cee3634/640/8a6555e5-8de6-4236-865f-19339cee3634.png)