303143
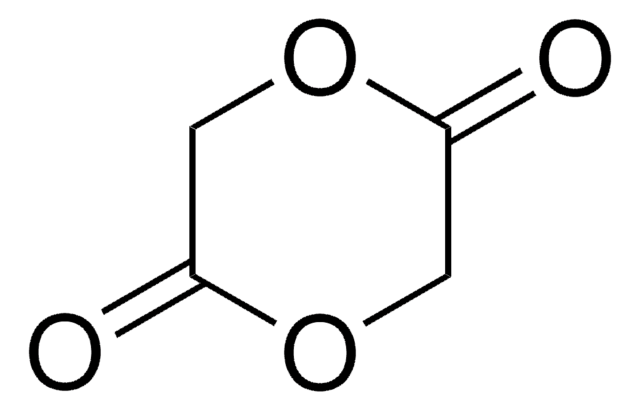
3,6-Dimethyl-1,4-dioxane-2,5-dione
99%
Synonym(s):
DL-Lactide, rac-Lactide, Lactide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H8O4
CAS Number:
Molecular Weight:
144.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
bp
142 °C/8 mmHg (lit.)
mp
116-119 °C
functional group
ester
storage temp.
2-8°C
SMILES string
CC1OC(=O)C(C)OC1=O
InChI
1S/C6H8O4/c1-3-5(7)10-4(2)6(8)9-3/h3-4H,1-2H3
InChI key
JJTUDXZGHPGLLC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3,6-Dimethyl-1,4-dioxane-2,5-dione (or rac-lactide), is the 50:50 racemic mixture of D- and L-Lactide. Rac-lactide is a lactone derived from lactic acid that has attracted great interest in academia and commercial applications, as it is derived from abundant renewable resources. Rac-lactide can be ready polymerized via ring-opening polymerization, using a variety of metal or organocatalysts, yielding poly(D,L-lactide). While the resulting polymer is generally amorphous, the use of stereospecific catalysts can lead to heterotactic PLA, which exhibits some degree of crystallinity.
Application
3,6-Dimethyl-1,4-dioxane-2,5-dione can be used as a reactant:
- To synthesize multi-block copolymers of polylactide and polycarbonate.
- In the aluminum-catalyzed polymerization of propene oxide, lactide, and phthalic anhydride to produce multi-block polyesters.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 47, 6517-6517 (2006)
Kimberly M Osten et al.
Dalton transactions (Cambridge, England : 2003), 41(26), 8123-8134 (2012-04-07)
Functionalized diaminophenols, H(N(R1R2)N(R3)O), were investigated as ligands for indium catalysts in the ring-opening polymerization of racemic lactide. Precursor complexes (N(Me2)N(Me)O)InCl(2) (1), (N(Pr2)NO)InCl(2) (2), and (N(Mes)NO)InCl(2) (3) were synthesized and fully characterized by (1)H and (13)C NMR spectroscopy, elemental analysis, and
Insun Yu et al.
Journal of the American Chemical Society, 134(30), 12758-12773 (2012-07-07)
A family of racemic and enantiopure indium complexes 1-11 bearing bulky chiral diaminoaryloxy ligands, H(NNO(R)), were synthesized and fully characterized. Investigation of both the mono- and the bis-alkoxy-bridged complexes [(NNO(R))InX](2)[μ-Y][μ-OEt] (5, R = (t)Bu, X = Y = Cl; 8
Anne Marit de Groot et al.
Journal of controlled release : official journal of the Controlled Release Society, 266, 27-35 (2017-09-18)
The skin is an attractive organ for immunization due to the presence of a large number of epidermal and dermal antigen-presenting cells. Hollow microneedles allow for precise and non-invasive intradermal delivery of vaccines. In this study, ovalbumin (OVA)-loaded poly(lactic-co-glycolic acid)
Clare Bakewell et al.
Journal of the American Chemical Society, 134(51), 20577-20580 (2012-12-13)
Highly active yttrium phosphasalen initiators for the stereocontrolled ring-opening polymerization of rac-lactide are reported. The initiators are coordinated by a new class of ancillary ligand: an iminophosphorane derivative of the popular "salen" ligand, termed "phosphasalen". Changing the phosphasalen structure enables
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service