334049
Gold(III) chloride
99%
Synonym(s):
Trichlorogold
About This Item
Recommended Products
Quality Level
Assay
99%
form
powder
reaction suitability
reagent type: catalyst
core: gold
SMILES string
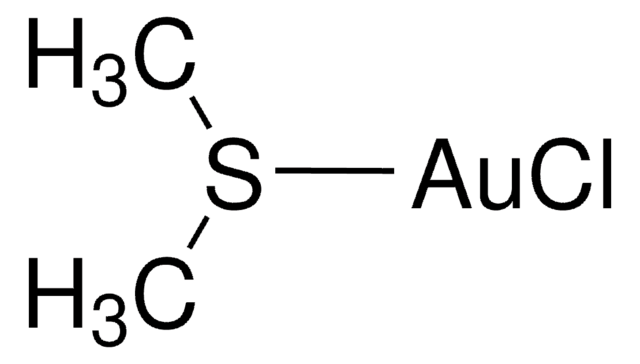
Cl[Au](Cl)Cl
InChI
1S/Au.3ClH/h;3*1H/q+3;;;/p-3
InChI key
RJHLTVSLYWWTEF-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a dopant to fabricate graphene protection layer between PEDOT:PSS and ITO for perovskite solar cells. It helps to enhance the photoconversion efficiency by protection ITO and collecting holes.
- To fabricate flexible and transparent electrodes for OLEDs. It fills the defects in graphene and improves current efficiency without affecting the transmittance.
- As a precursor for green synthesis of gold nanoparticles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Noble-metal nanostructures find diverse applications from catalysis to biomedical research, leveraging surface properties in various fields.
Plasmonic nanoparticles have unique optical properties that can be tailored to suit a variety of applications in the biotechnology1–8 and electronics9–16 industries.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service