338257
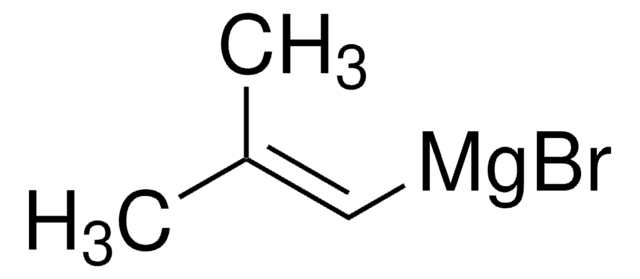
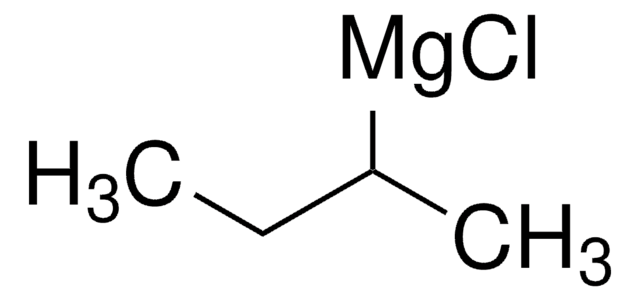
Isobutylmagnesium bromide solution
2.0 M in diethyl ether
Synonym(s):
iBuMgBr solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHCH2MgBr
CAS Number:
Molecular Weight:
161.32
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
2.0 M in diethyl ether
density
0.941 g/mL at 25 °C
SMILES string
CC(C)C[Mg]Br
InChI
1S/C4H9.BrH.Mg/c1-4(2)3;;/h4H,1H2,2-3H3;1H;/q;;+1/p-1
InChI key
CMWBEISSZHZIMU-UHFFFAOYSA-M
Related Categories
Application
Isobutylmagnesium bromide (iBuMgBr) is a general Grignard reagent used in the total synthesis of (+)-rishirilide B, glucolipsin A, and (+)-juvabione. It can also be used as a reagent in the synthesis of pyrrolidine-based influenza neuraminidase (NA) inhibitors.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
-29.2 °F - closed cup
Flash Point(C)
-34 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Total synthesis of (+)-rishirilide B: Development and application of general processes for enantioselective oxidative dearomatization of resorcinol derivatives.
Mejorado LH and Pettus TRR
Journal of the American Chemical Society, 128(49), 15625-15631 (2006)
Structure-based characterization and optimization of novel hydrophobic binding interactions in a series of pyrrolidine influenza neuraminidase inhibitors.
Maring CJ, et al.
Journal of Medicinal Chemistry, 48(12), 3980-3990 (2005)
Structure assignment, total synthesis, and evaluation of the phosphatase modulating activity of glucolipsin A.
Furstner A, et al.
The Journal of Organic Chemistry, 69(2), 459-467 (2004)
Enantio-and diastereocontrolled synthesis of (+)-juvabione employing organocatalytic desymmetrisation and photoinduced fragmentation.
Itagaki N and Iwabuchi Y
Chemical Communications (Cambridge, England), 69(11), 1175-1176 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service