363340
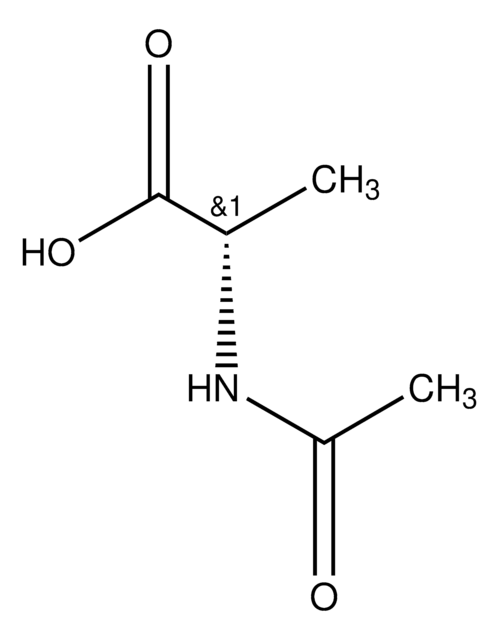
N-Acetylcysteamine
95%
Synonym(s):
N-(2-Mercaptoethyl)acetamide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3CONHCH2CH2SH
CAS Number:
Molecular Weight:
119.19
MDL number:
UNSPSC Code:
12352100
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.511 (lit.)
bp
138-140 °C/7 mmHg (lit.)
mp
6-7 °C (lit.)
density
1.121 g/mL at 25 °C (lit.)
functional group
amide
thiol
SMILES string
CC(=O)NCCS
InChI
1S/C4H9NOS/c1-4(6)5-2-3-7/h7H,2-3H2,1H3,(H,5,6)
InChI key
AXFZADXWLMXITO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-Acetylcysteamine, also known as N-(2-Mercaptoethyl) acetamide, is a derivative of cysteamine, that is commonly used as a building block for the synthesis of alkylated thiol and thioesters via esterification.
Application
N-Acetylcysteamine can be used as a building block to synthesize:
- N
- -acetylcysteamine (SNAC) thioesters by reacting with various acid derivatives in the presence of 1,1′-carbonyldiimidazole (CDI).
- Thieno[2,3-c]pyrrole derivatives via three-component reaction of 2-acetyl-3-thiophenecarboxaldehyde and various amines.
- Carbapenems, a class of beta-lactam antibiotic agents.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 29, 4305-4305 (1988)
Thomas Frenzel et al.
Organic letters, 8(1), 135-138 (2005-12-31)
[structure: see text] The enantioselective total synthesis of the N-acetylcysteamine thioester of seco-proansamitocin, a key biosynthetic intermediate of the highly potent antitumor agent ansamitocin, is described, which twice utilizes the Nagao acetate aldol reaction, as well as an indium-mediated alkynylation
Cynthia M Hong et al.
Organic & biomolecular chemistry, 11(18), 2932-2935 (2013-03-29)
A three-component reaction has been developed that allows the regioselective synthesis of thieno[2,3-c]pyrroles. The reaction is based on the ability of 2-acetyl-3-thiophenecarboxaldehyde to react with amine and thiol nucleophiles to produce the corresponding tri-substituted thieno[2,3-c]pyrroles, with water as the only
S A Moore et al.
The Journal of biological chemistry, 257(18), 10874-10881 (1982-09-25)
Succinyl phosphate reacts rapidly with the thiol N-acetyl-beta-mercaptoethylamine (a CoA analog) to form the succinate thiol ester. This is a chemical model for the enzymic reaction of CoA transferase. It is demonstrated that: 1) the chemical reaction is stepwise, proceeding
Magoichi Sako et al.
The Journal of organic chemistry, 63(20), 6947-6951 (2001-10-24)
4H-[1,2,5]Oxadiazolo[3,4-d]pyrimidine-5,7-dione 1-oxides (2) are conveniently prepared in high yields by the oxidative intramolecular cyclization of 6-amino-5-nitro-1H-pyrimidine-2,4-diones (1) employing iodosylbenzene diacetate as an oxidant in the presence of lithium hydride. The generation of nitric oxide (NO) and NO-related species from 2
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service