37275
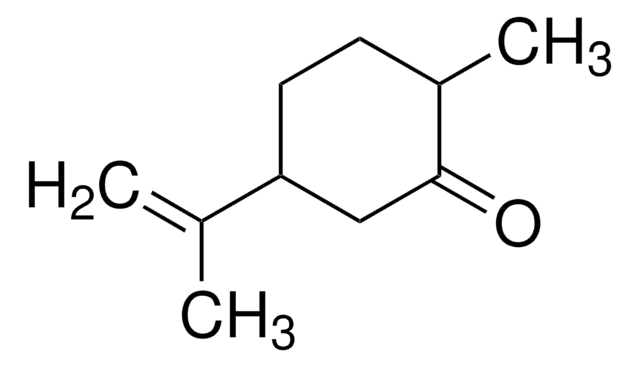
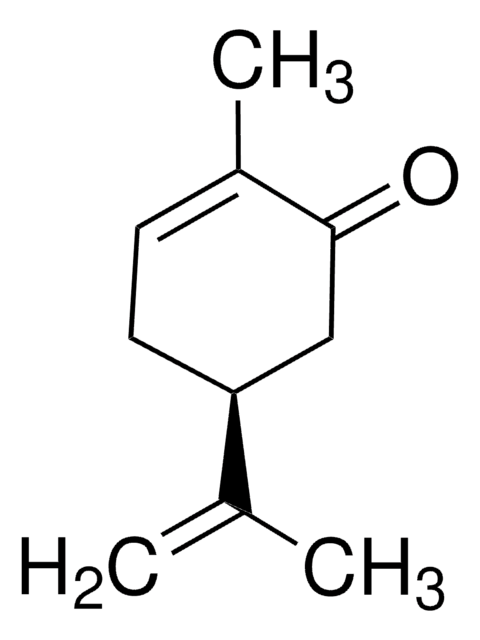
(+)-Dihydrocarvone
mixture of isomers
Synonym(s):
(2R,5R)-5-Isopropenyl-2-methylcyclohexanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H16O
CAS Number:
Molecular Weight:
152.23
Beilstein:
2044615
EC Number:
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
form
liquid
optical activity
[α]20/D +20±2°, neat
composition
n-(+)-dihydrocarvone, ~77%
iso-(+)-dihydrocarvone, ~20%
refractive index
n20/D 1.471
density
0.928 g/mL at 20 °C (lit.)
functional group
ketone
SMILES string
CC1CCC(CC1=O)C(C)=C
InChI
1S/C10H16O/c1-7(2)9-5-4-8(3)10(11)6-9/h8-9H,1,4-6H2,2-3H3
InChI key
AZOCECCLWFDTAP-UHFFFAOYSA-N
Related Categories
General description
(+)-Dihydrocarvone, a monoterpenoid compound found in caraway oil, is a key building block to synthesize sesquiterpenes. It is generally produced either by the hydrogenation of carvone or oxidation of limonene.
Application
(+)-Dihydrocarvone may be used in the following processes:
- Synthesis of dispiro 1,2,4,5-tetraoxanes, which show potent anti-malarial activity.
- Synthesis of an epoxylactone by oxidation, which can undergo copolymerization with ε-caprolactone to form cross-linked copolymers with shape memory properties.
- Synthesis of α-Cyperone, a eudesmane type sesquiterpenoid compound with potent insecticidal activity.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
215.6 °F - closed cup
Flash Point(C)
102 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dan Jin et al.
Scientific reports, 10(1), 3309-3309 (2020-02-26)
Cannabis research has historically focused on the most prevalent cannabinoids. However, extracts with a broad spectrum of secondary metabolites may have increased efficacy and decreased adverse effects compared to cannabinoids in isolation. Cannabis's complexity contributes to the length and breadth
Oxidized dihydrocarvone as a renewable multifunctional monomer for the synthesis of shape memory polyesters.
Lowe JR, et al.
Biomacromolecules, 10(7), 2003-2008 (2009)
The structure and antimalarial activity of dispiro-1, 2, 4, 5-tetraoxanes derived from (+)-dihydrocarvone.
Dong Y, et al.
Bioorganic & Medicinal Chemistry Letters, 20(22), 6359-6361 (2010)
Insecticidal activity of sesquiterpenes skeleton synthesized by the conventional Robinson annulations reaction on Drosophila melanogaster.
Alarcon J, et al.
Industrial Crops and Products, 42, 268-272 (2013)
N-functionalization of dihydrocarvone: Obtaining aminocyclohexane derivatives and their spectrometric study.
Kouznetsov VV and Stashenko EE.
Journal of the Chilean Chemical Society, 50(3), 559-563 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service