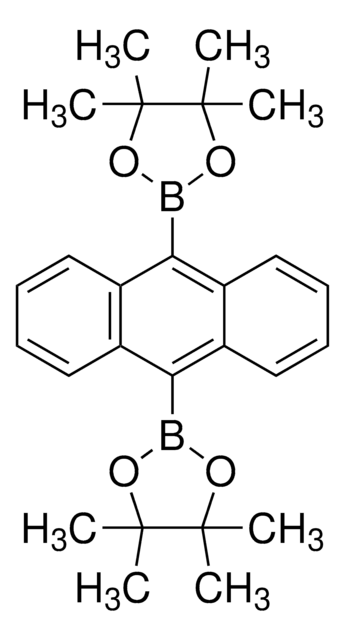
459852
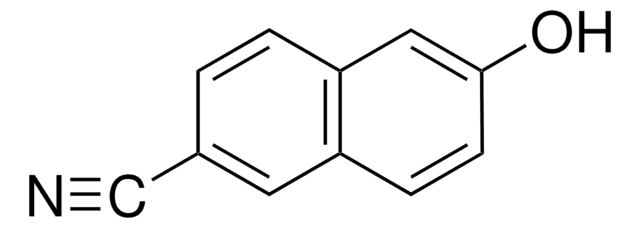
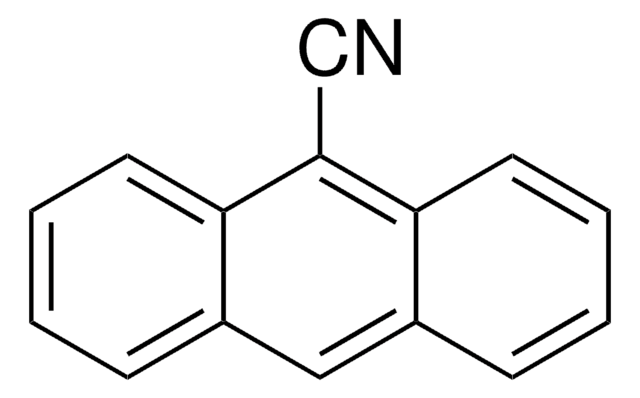
9,10-Anthracenedicarbonitrile
97%
Synonym(s):
9,10-Dicyanoanthracene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H8N2
CAS Number:
Molecular Weight:
228.25
Beilstein:
1646384
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
340 °C (lit.)
functional group
nitrile
SMILES string
N#Cc1c2ccccc2c(C#N)c3ccccc13
InChI
1S/C16H8N2/c17-9-15-11-5-1-2-6-12(11)16(10-18)14-8-4-3-7-13(14)15/h1-8H
InChI key
BIOPPFDHKHWJIA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
9,10-Anthracenedicarbonitrile (DCA, ADC) is an anthracene derivative. Photochemical reactions of DCA in MeCN-MeOH or MeCN-H2O containing hydroxides or methoxides affords 9-methylimino-10-anthracenecarbonitrile. It participates in the generation of nucleophilic α-hydroxymethyl radicals from α-silyl ethers by irradiation. Binding energy of 9,10-anthracenedicarbonitrile adsorbed onto graphene is -1.23eV.
Application
9,10-Anthracenedicarbonitrile may be used as photosensitizer to investigate the photoisomerization of trans-stilbene (TS) in sodium dodecyl sulfate (SDS)/benzyl alcohol (BA)/H2O system.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Photoinduced Bimolecular Electron Transfer from Cyano Anions in Ionic Liquids.
Boning W, et al.
The Journal of Physical Chemistry B, 119, 14790-14799 (2015)
a-Silyl Ethers as Hydroxymethyl Anion Equivalents in Photoinduced Radical Electron Transfer Additions.
Gutenberger G, et al.
Angewandte Chemie (International Edition in English), 37(5), 660-662 (1998)
Xia Guo et al.
Journal of colloid and interface science, 283(2), 578-584 (2005-02-22)
Photoisomerization of trans-stilbene (TS) was investigated in sodium dodecyl sulfate (SDS)/benzyl alcohol (BA)/H(2)O systems in order to establish the relationship between the reaction yields and the compositions and structures of molecular organized assemblies. The results show that, in SDS/BA/H(2)O systems
The photochemical reactions of 9, 10-anthracenedicarbonitrile and 1, 4-naphthalenedicarbonitrile in acetonitrile in the presence of bases.
Freccero M, et al.
Tetrahedron, 50(7), 2115-2130 (1994)
Steven Bailey et al.
The Journal of chemical physics, 140(5), 054708-054708 (2014-02-12)
To identify families of stable planar anchor groups for use in single molecule electronics, we report detailed results for the binding energies of two families of anthracene and pyrene derivatives adsorbed onto graphene. We find that all the selected derivatives
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)