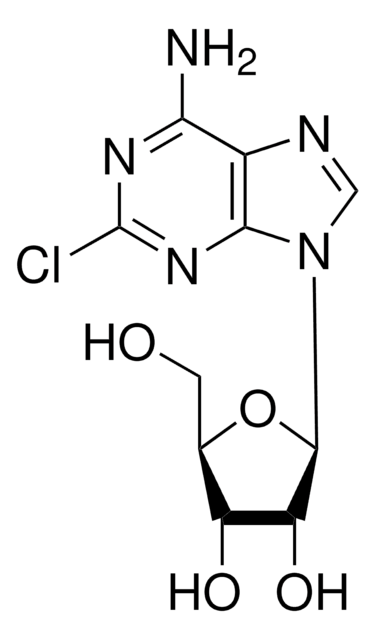
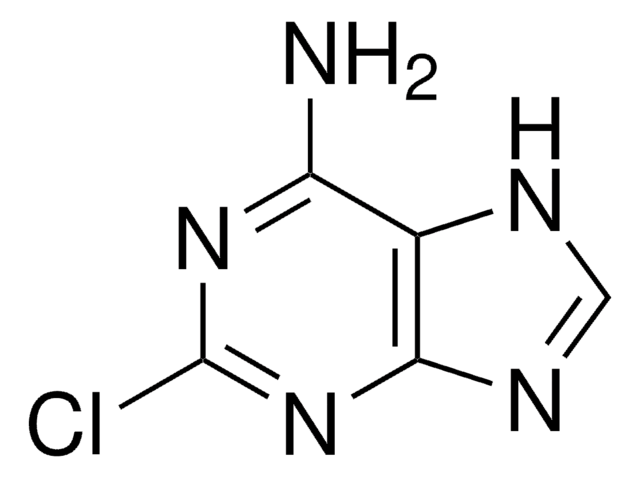
535087
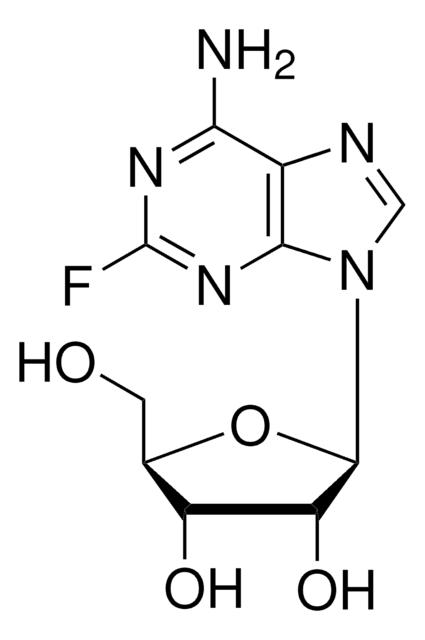
2-Fluoroadenine
96%
Synonym(s):
2-Fluoro-7(9)H-purin-6-ylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H4FN5
CAS Number:
Molecular Weight:
153.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
>350 °C (lit.)
SMILES string
Nc1[nH]c(F)nc2ncnc12
InChI
1S/C5H4FN5/c6-5-10-3(7)2-4(11-5)9-1-8-2/h1H,(H3,7,8,9,10,11)
InChI key
WKMPTBDYDNUJLF-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
V I Avramis et al.
Biochemical and biophysical research communications, 113(1), 35-43 (1983-05-31)
Murine P388 cells incubated in vitro with the anticancer drug arabinosyl 2-fluoroadenine accumulate its 5'-triphosphate, F-araATP, as the major phosphorylated metabolite. A new chromatographically separate metabolite that accumulated to levels 10% of that of F-araATP was identified as 2-fluoro-ATP, by
Sepideh Afshar et al.
Protein science : a publication of the Protein Society, 18(5), 1107-1114 (2009-04-24)
A double mutant of human purine nucleoside phosphorylase (hDM) with the amino acid mutations Glu201Gln:Asn243Asp cleaves adenosine-based prodrugs to their corresponding cytotoxic drugs. When fused to an anti-tumor targeting component, hDM is targeted to tumor cells, where it effectively catalyzes
Lincoln G Scott et al.
Journal of the American Chemical Society, 126(38), 11776-11777 (2004-09-24)
The production of isotopically labeled RNA remains critical to current NMR structural studies. One approach to obtain simple NMR spectra is to label with a nucleus that is not naturally occurring in RNA. Fluorine-19 can serve as a sensitive site-specific
P Huang et al.
Biochemical pharmacology, 36(18), 2945-2950 (1987-09-15)
2-Fluoroadenine (F-Ade) is a metabolite of 9-beta-D-arabinofuranosyl-2-fluoroadenine (F-ara-A) that may be involved in the development of toxic side effects from this anticancer drug. The liberation of F-Ade from F-ara-A has been examined in different biological systems. Extracts of Escherichia coli
Yukio Kitade et al.
Nucleic acids research. Supplement (2001), (3)(3), 5-6 (2003-09-27)
Carbocyclic and acyclic nucleosides possessing 2-fluoroadenine, such as 2-fluoronoraristeromycin (6) and 2-fluoro-9-[(2S,3R)-2,3,4-trihydroxy-butyl-1-yl]adenine (8), were synthesized and their inhibitory activities against human and Plasmodium falciparum recombinant SAH hydrolase were investigated.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service