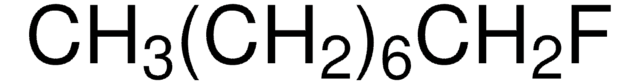538647
1-Fluorododecane
98%
Synonym(s):
n-Dodecyl fluoride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
F(CH2)11CH3
CAS Number:
Molecular Weight:
188.33
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.42 (lit.)
density
0.807 g/mL at 25 °C (lit.)
functional group
alkyl halide
fluoro
SMILES string
CCCCCCCCCCCCF
InChI
1S/C12H25F/c1-2-3-4-5-6-7-8-9-10-11-12-13/h2-12H2,1H3
InChI key
YHYBNVZCQIDLSQ-UHFFFAOYSA-N
General description
1-Fluorododecane is a long chain 1-fluoroalkane. It can be synthesized from the reaction between 1-dodecanol, N,N-diethyl-α,α-difluoro-[3,5-bis(1H,1H,2H,2H-perfluorodecyl)benzyl]amine and heptane. 1-Fluorododecane undergoes reaction with dibromomethane and titanocene dichloride in the presence of triethyl aluminium (Et3Al) to afford a mixture of 1-chlorododecane and 1-bromododecane. 1-Fluorododecane can also be prepared from 1-hydroxydodecane.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Aquatic Chronic 4
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
223.0 °F - closed cup
Flash Point(C)
106.1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Halogen Exchange Reaction of Aliphatic Fluorine Compounds with Organic Halides as Halogen Source.
Mizukami Y, et al.
Organic Letters, 17(24), 5942-5945 (2015)
Regioselective Hydroxylation of C12?C15 Fatty Acids with Fluorinated Substituents by Cytochrome P450 BM3.
Chiang, Chih-Hsiang, et al.
Chemistry?A European Journal , 19(41), 13680-13691 (2013)
Fluorination of alcohols and diols with a novel fluorous deoxy-fluorination reagent.
Furuya T, et al.
Journal of Fluorine Chemistry, 130(2), 348-353 (2009)
Vapor Pressures and Boiling Points of the 1-Fluoroalkanes, 1-Chloroalkanes, 1-Bromoalkanes, and 1-Iodoalkanes, C1to C20.
Li JCM and Rossini RD.
Journal of Chemical and Engineering Data, 6(2), 268-270 (1961)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service