557161
Bis(4-chlorophenyl) disulfide
97%
Synonym(s):
4,4′-Dichlorodiphenyl disulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
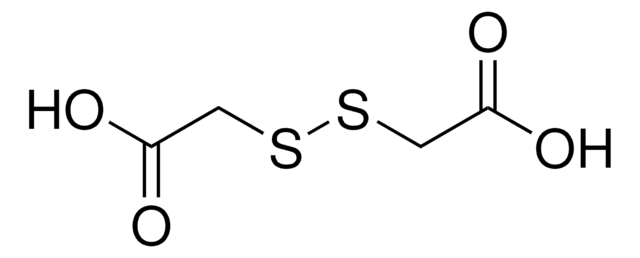
Linear Formula:
[ClC6H4S]2
CAS Number:
Molecular Weight:
287.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
71-74 °C (lit.)
functional group
chloro
disulfide
SMILES string
Clc1ccc(SSc2ccc(Cl)cc2)cc1
InChI
1S/C12H8Cl2S2/c13-9-1-5-11(6-2-9)15-16-12-7-3-10(14)4-8-12/h1-8H
InChI key
ZIXXRXGPBFMPFD-UHFFFAOYSA-N
General description
Bis(4-chlorophenyl) disulphide can be synthesized from 4-chlorophenylthiol via oxidation. It produces poly(p-phenylene sulfide), via thermolysis. Bis(4-chlorophenyl) disulfide can also be prepared by a microwave assisted method involving the reaction between respective elemental sulfur and 1-chloro-4-iodobenzene in the presence of CuO nanopowder (catalyst).
Application
Bis(4-chlorophenyl) disulfide may be used to synthesize non-symmetrical heterodimer 4-chlorophenyl-2′-nitrophenyl disulfide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Microwave-assisted one-pot synthesis of symmetrical diselenides, ditellurides and disulfides from organoyl iodides and elemental chalcogen catalyzed by CuO nanoparticles.
Botteselle GV, et al.
J. Mol. Catal. A: Chem., 365, 186-193 (2012)
Synthesis of poly (p-phenylene sulfide) by thermolysis of bis (4-halophenyl) disulfides.
Wang ZY and Hay AS.
Macromolecules, 24(1), 333-335 (1991)
A mild and environmentally benign oxidation of thiols to disulfides.
Kirihara M, et al.
Synthesis, 2007(21), 3286-3289 (2007)
Szymon Sobczak et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 24(35), 8769-8773 (2018-04-21)
This work describes, for the first time, the application of combined pressure and temperature stimuli in disulfide metathesis reactions. In the system studied, above a pressure of 0.2 GPa, equimolar amounts of symmetric disulfides bis 4-chlorophenyl disulfide [(4-ClPhS)2 ] and bis
Tomislav Friščić
Chemical Society reviews, 41(9), 3493-3510 (2012-03-01)
Mechanochemical reactions effected by milling or grinding are an attractive means to conduct chemical reactions dependent on molecular recognition and to systematically explore different modes of molecular self-assembly. The natural relationship between milling mechanochemistry and supramolecular chemistry arises primarily from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service