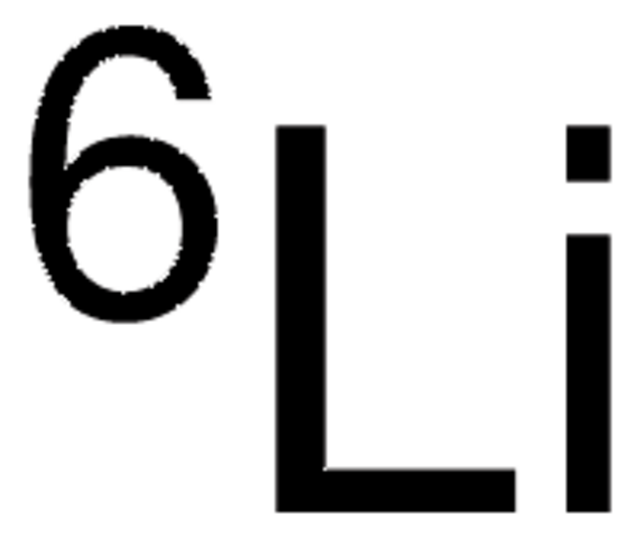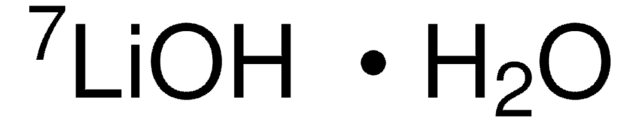All Photos(1)
About This Item
Empirical Formula (Hill Notation):
7Li
CAS Number:
Molecular Weight:
7.02
UNSPSC Code:
12141803
PubChem Substance ID:
Recommended Products
isotopic purity
≥99.8 atom % 7Li
Assay
≥99.8% (CP)
form
solid
mass shift
depleted
SMILES string
[7Li]
InChI
1S/Li
InChI key
WHXSMMKQMYFTQS-UHFFFAOYSA-N
Related Categories
Packaging
This product may be available from bulk stock and can be packaged on demand. For information on pricing, availability and packaging, please contact Stable Isotopes Customer Service.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B - Water-react 1
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xiao Tang et al.
Scientific reports, 5, 11958-11958 (2015-07-08)
LiNi0.5Mn1.5O4 nanorods wrapped with graphene nanosheets have been prepared and investigated as high energy and high power cathode material for lithium-ion batteries. The structural characterization by X-ray diffraction, Raman spectroscopy, and Fourier transform infrared spectroscopy indicates the LiNi0.5Mn1.5O4 nanorods prepared
Mohamed Aklalouch et al.
ChemSusChem, 8(20), 3465-3471 (2015-09-19)
By comparing carbon electrodes with varying porosity in Li-O2 cells, we show that the effect of electrolyte stirring at a given current density can result in a change from 2D to 3D growth of discharged deposits. The change of morphology
Emanuel Peled et al.
Nano letters, 15(6), 3907-3916 (2015-05-15)
Here, we report on the scalable synthesis and characterization of novel architecture three-dimensional (3D) high-capacity amorphous silicon nanowires (SiNWs)-based anodes with focus on studying their electrochemical degradation mechanisms. We achieved an unprecedented combination of remarkable performance characteristics, high loadings of
M Helen et al.
Scientific reports, 5, 12146-12146 (2015-07-16)
Lithium-sulphur batteries have generated tremendous research interest due to their high theoretical energy density and potential cost-effectiveness. The commercial realization of Li-S batteries is still hampered by reduced cycle life associated with the formation of electrolyte soluble higher-order polysulphide (Li2Sx
Jennifer Nicholas et al.
Journal of medical Internet research, 17(8), e198-e198 (2015-08-19)
With continued increases in smartphone ownership, researchers and clinicians are investigating the use of this technology to enhance the management of chronic illnesses such as bipolar disorder (BD). Smartphones can be used to deliver interventions and psychoeducation, supplement treatment, and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service