653322
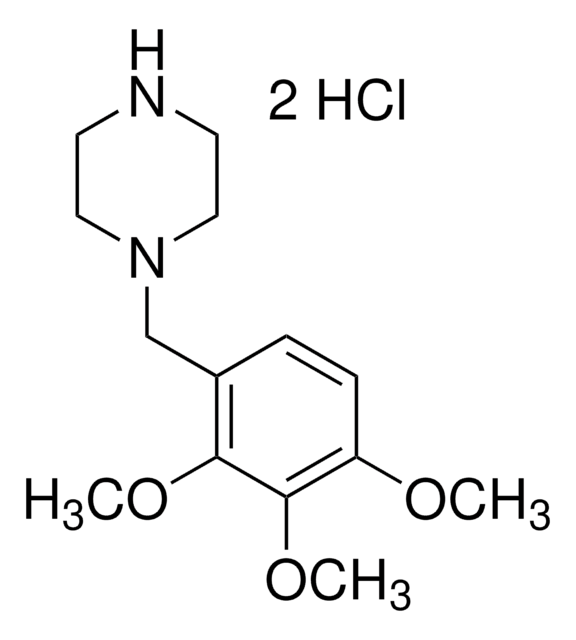
1-(2,3,4-Trimethoxybenzyl)piperazine dihydrochloride
97%
Synonym(s):
Trimetazidine dihydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C14H22N2O3 · 2HCl
CAS Number:
Molecular Weight:
339.26
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
231-235 °C (lit.)
SMILES string
Cl[H].Cl[H].COc1ccc(CN2CCNCC2)c(OC)c1OC
InChI
1S/C14H22N2O3.2ClH/c1-17-12-5-4-11(13(18-2)14(12)19-3)10-16-8-6-15-7-9-16;;/h4-5,15H,6-10H2,1-3H3;2*1H
InChI key
VYFLPFGUVGMBEP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-(2,3,4-Trimethoxybenzyl)piperazine dihydrochloride or trimetazidine dihydrochloride can be used as a building block to synthesize:
- Phenylpropyl trimetazidine derivatives with potent cerebral vasodilator activity.
- Benzoylguanidine-trimetazidine derivatives for myocardial ischemic-reperfusion activity studies.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Design, synthesis and biological evaluation of novel substituted benzoylguanidine derivatives as potent Na+/H+ exchanger inhibitors
Xu W-T, et al.
Bioorganic & Medicinal Chemistry Letters, 19(12), 3283-3287 (2009)
Benzylpiperazine Derivatives. I. Syntheses and Biological Activities of 1 (2, 3, 4-Trimethoxybenzyl) piperazine Derivatives
OHTAKA H, et al.
Chemical & Pharmaceutical Bulletin, 35(7), 2774-2781 (1987)
Le Tran Phuc Khoa et al.
Cell stem cell, 27(3), 441-458 (2020-07-02)
Self-renewing embryonic stem cells (ESCs) respond to environmental cues by exiting pluripotency or entering a quiescent state. The molecular basis underlying this fate choice remains unclear. Here, we show that histone acetyltransferase MOF plays a critical role in this process
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service