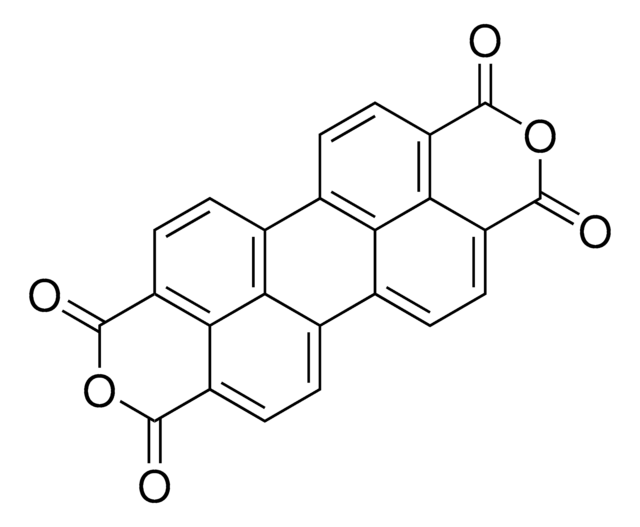
663913
N,N′-Dioctyl-3,4,9,10-perylenedicarboximide
98%
Synonym(s):
PTCDI-C8
About This Item
Recommended Products
Quality Level
Assay
98%
form
solid
mp
>300 °C
λmax
526 nm
fluorescence
λem ≤533 nm in chloroform
semiconductor properties
N-type (mobility=1.7 cm2/V·s)
SMILES string
CCCCCCCCN1C(=O)c2ccc3c4ccc5C(=O)N(CCCCCCCC)C(=O)c6ccc(c7ccc(C1=O)c2c37)c4c56
InChI
1S/C40H42N2O4/c1-3-5-7-9-11-13-23-41-37(43)29-19-15-25-27-17-21-31-36-32(40(46)42(39(31)45)24-14-12-10-8-6-4-2)22-18-28(34(27)36)26-16-20-30(38(41)44)35(29)33(25)26/h15-22H,3-14,23-24H2,1-2H3
InChI key
YFGMQDNQVFJKTR-UHFFFAOYSA-N
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Flexible electronic circuits, displays, and sensors based on organic active materials will enable future generations of electronics products that may eventually enter the mainstream electronics market.
Review the potential of self-assembled multilayer gate dielectric films fabricated from silane precursors for organic, inorganic, and transparent TFT and for TFT circuitry and OLED displays.
Small molecular weight organic semiconductors are promising for flexible transistor applications in next-gen soft electronics.
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service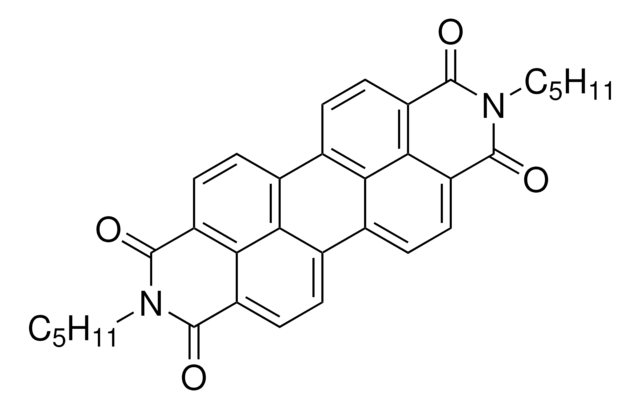
![4,9-Dibromo-2,7-bis(2-octyldodecyl)benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone](/deepweb/assets/sigmaaldrich/product/structures/100/693/87f6ccae-153b-4ba6-bd0a-653ba06ece22/640/87f6ccae-153b-4ba6-bd0a-653ba06ece22.png)
![N,N′-Bis[2-(2-tert-butyldimethylsilyloxyethoxy)ethyl]-3,4,9,10-perylenetetracarboxylic diimide 97%](/deepweb/assets/sigmaaldrich/product/structures/334/047/4ac691aa-ae25-4df1-9e0d-09ed12cb8f1f/640/4ac691aa-ae25-4df1-9e0d-09ed12cb8f1f.png)