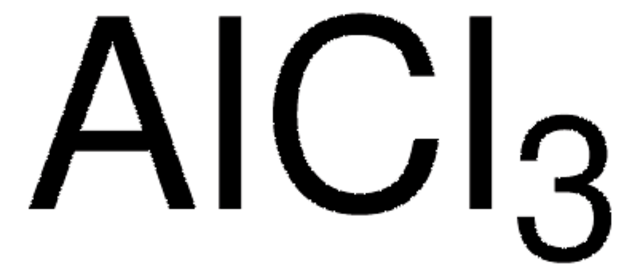678732
Boron trichloride solution
1.0 M in toluene
Synonym(s):
Boron chloride, Trichloroborane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
BCl3
CAS Number:
Molecular Weight:
117.17
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
concentration
1.0 M in toluene
density
0.909 g/mL at 25 °C
SMILES string
ClB(Cl)Cl
InChI
1S/BCl3/c2-1(3)4
InChI key
FAQYAMRNWDIXMY-UHFFFAOYSA-N
General description
Boron trichloride (BCl3) is a boron halide typically used as a reagent in organic synthesis for the cleavage of C-O bonds in ethers.
Application
Boron trichloride (BCl3) can be used as a reactant in the preperation of borazine-linked polymer [BLP-10(Cl)] by thermal decomposition with benzidine for gas storage and purification.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Inhalation - Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 1B - Skin Corr. 1B - STOT RE 2 - STOT SE 3
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-2.0 °F
Flash Point(C)
-18.9 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Highly selective CO 2/CH 4 gas uptake by a halogen-decorated borazine-linked polymer
Reich TE, et al.
Journal of Materials Chemistry, 22(27), 13524-13528 (2012)
Conversion of aromatic aldehydes to gem-dichlorides using boron trichloride. A new highly efficient method for preparing dichloroarylmethanes.
Kabalka G W and Wu Z
Tetrahedron Letters, 41(5), 579-581 (2000)
Juan Xie et al.
Carbohydrate research, 340(3), 481-487 (2005-02-01)
Boron trichloride has been found to promote selective deprotection of 1,2- or 1,3-cis oriented secondary benzyl ethers of per-benzylated C-glycosyl derivatives. The reactivity towards BCl(3) follows the order: C-4>or=C-2>C-6>C-3 for C-glucopyranosyl derivatives and C-3>or=C-4>C-6>C-2 for C-galactopyranosyl derivatives. Preparatively useful selective
Jayaraman Selvakumar et al.
Organic & biomolecular chemistry, 8(18), 4056-4058 (2010-07-29)
Isoindoloisoquinalinone, pyrroloisoquinolinone and benzo[a]quinolizinone units are constructed via intramolecular cyclization of the methoxy substituted N-phenethylimides using BBr(3).
George W Kabalka et al.
The Journal of organic chemistry, 73(7), 2668-2673 (2008-03-06)
A boron trihalide mediated alkyne-aldehyde coupling reaction leading to stereodefined 1,3,5-triaryl-1,5-dihalo-1,4-pentadienes is described. The study led to the discovery of a direct substitution of hydroxyl groups by stereodefined alkenyl moieties using alkenylboron dihalides. During the investigation, it was also discovered
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service