733539
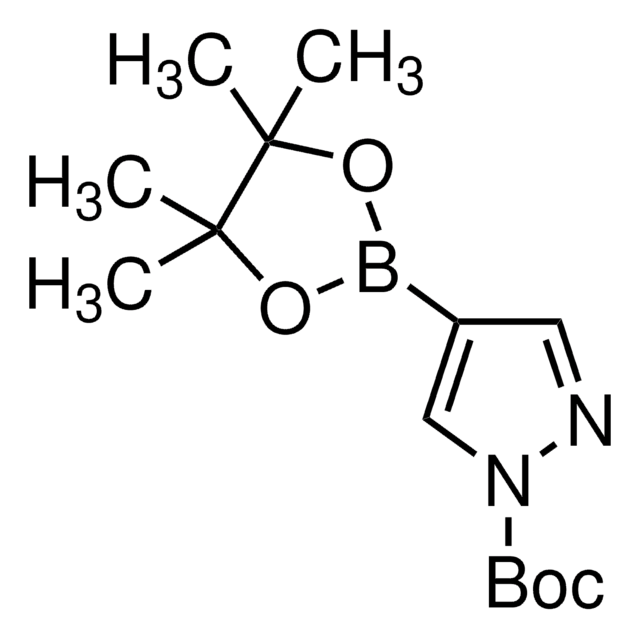
N-Boc-pyrrole-2-boronic acid MIDA ester
95%
Synonym(s):
1-(tert-Butoxycarbonyl)pyrrole-2-boronic acid MIDA ester
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C14H19BN2O6
CAS Number:
Molecular Weight:
322.12
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
166-171 °C
SMILES string
CN1CC(=O)OB(OC(=O)C1)c2cccn2C(=O)OC(C)(C)C
InChI
1S/C14H19BN2O6/c1-14(2,3)21-13(20)17-7-5-6-10(17)15-22-11(18)8-16(4)9-12(19)23-15/h5-7H,8-9H2,1-4H3
InChI key
HASMDYSWFBSATG-UHFFFAOYSA-N
Related Categories
Application
N-Boc-pyrrole-2-boronic acid MIDA ester can be used:
- As a starting material for the synthesis of marine natural product pentabromopseudilin.
- To prepare 5,5′-(3,4-dihexylthiophene-2,5-diyl)bis(1H-pyrrole-2-carbaldehyde), a key intermediate for the synthesis of thiophene based novel macrocycles.
- As a substrate in the palladium-catalyzed one-pot meta arylation of bromo substituted 2-phenylpyridine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Quaterpyrroles as building blocks for the synthesis of expanded porphyrins
Anguera G, et al.
Organic Letters, 17(9), 2194-2197 (2015)
Two-step total synthesis of an anti-MRSA and myosin-inhibiting marine natural product pentabromopseudilin via Suzuki-Miyaura coupling of a MIDA boronate ester
Kum D, et al.
Tetrahedron Letters, 58(34), 3374-3376 (2017)
Ruthenium-Catalyzed meta-Selective C-H Bromination
Teskey CJ, et al.
Angewandte Chemie (International Edition in English), 54(40), 11677-11680 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)

![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)